Activation Energy Drawing
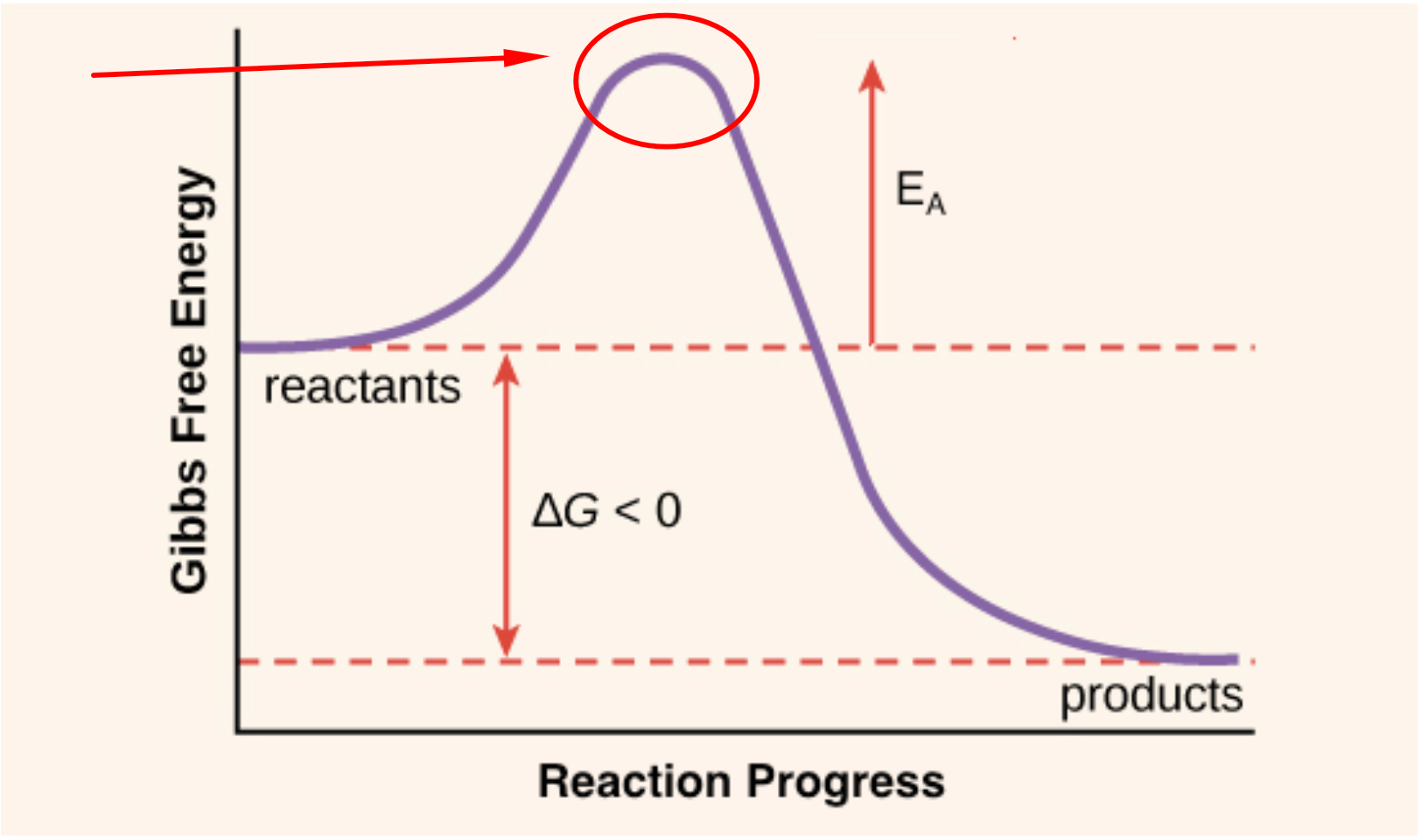
Activation Energy Drawing - The activation energy (e a) of a reaction is measured in kilojoules per mole (kj/mol) or kilocalories per mole (kcal/mol). Taking log on both sides. Web solution we can obtain the activation energy by plotting ln k versus , knowing that the slope will be equal to. Web the activation energy is what determines the kinetics of a reaction: Web the activation energy shown in the diagram below is for the forward reaction (reactants → products), which is exergonic.
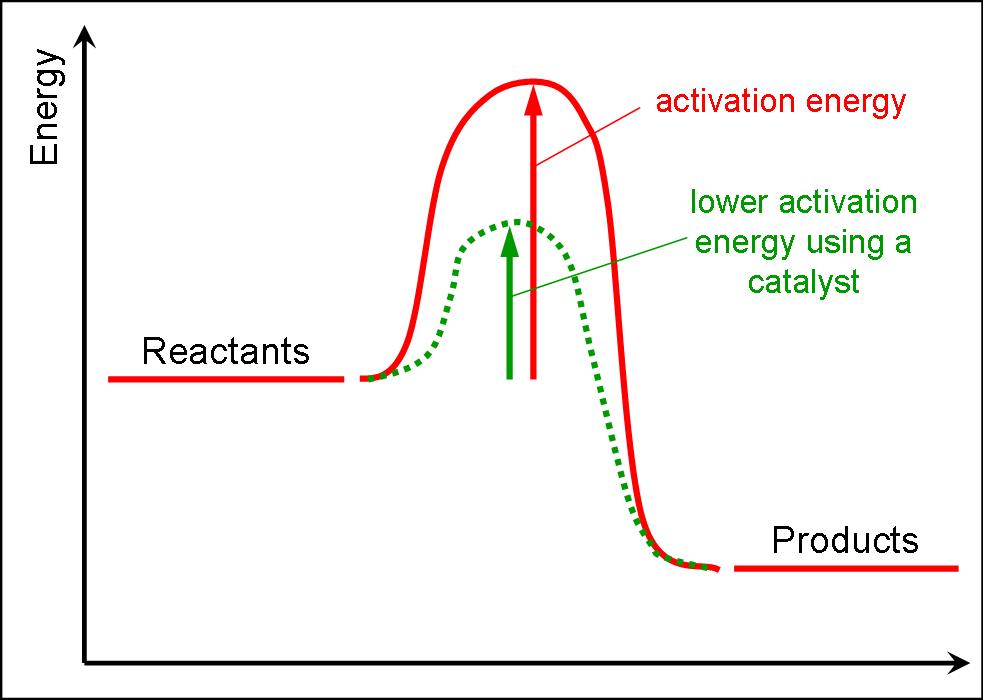
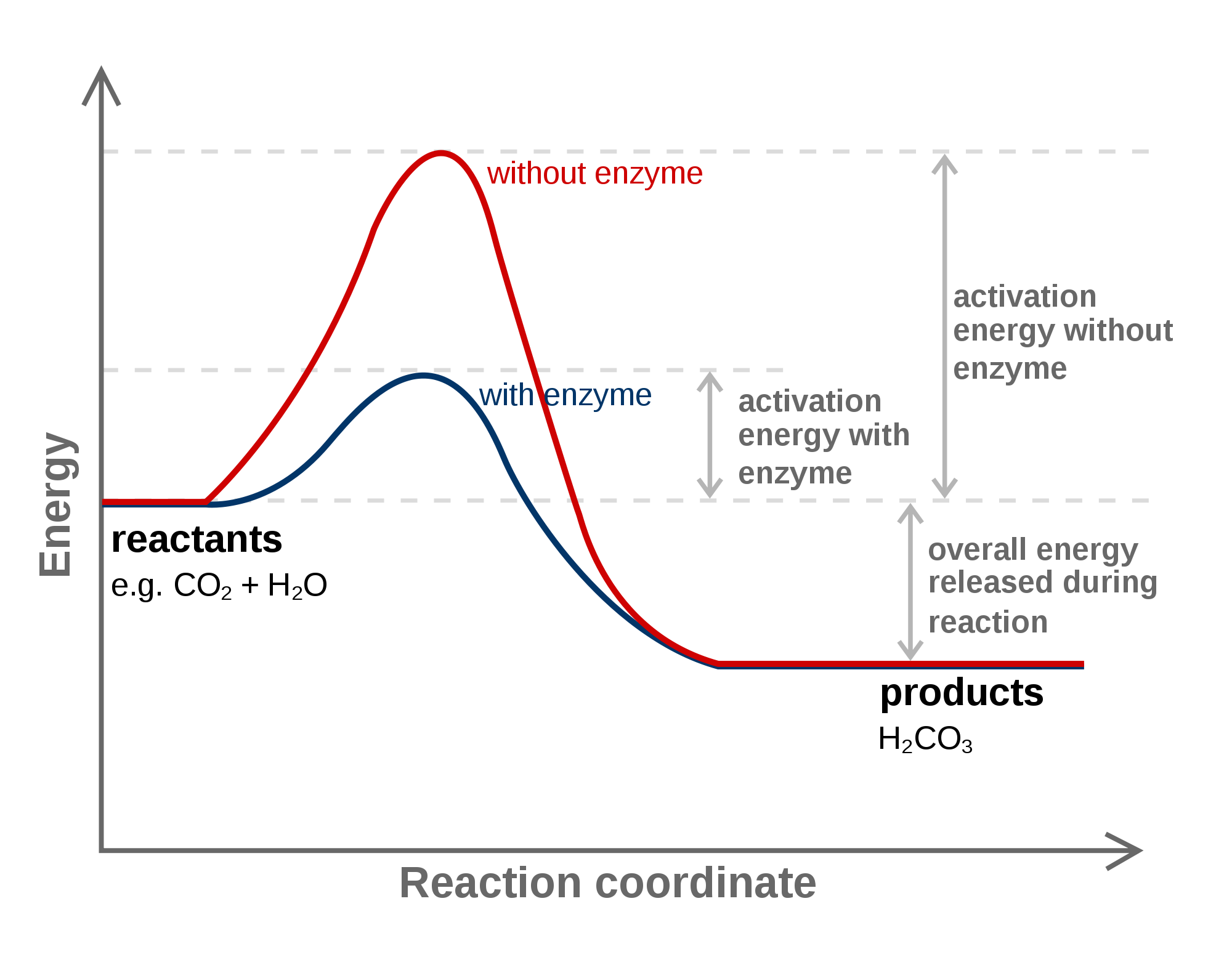
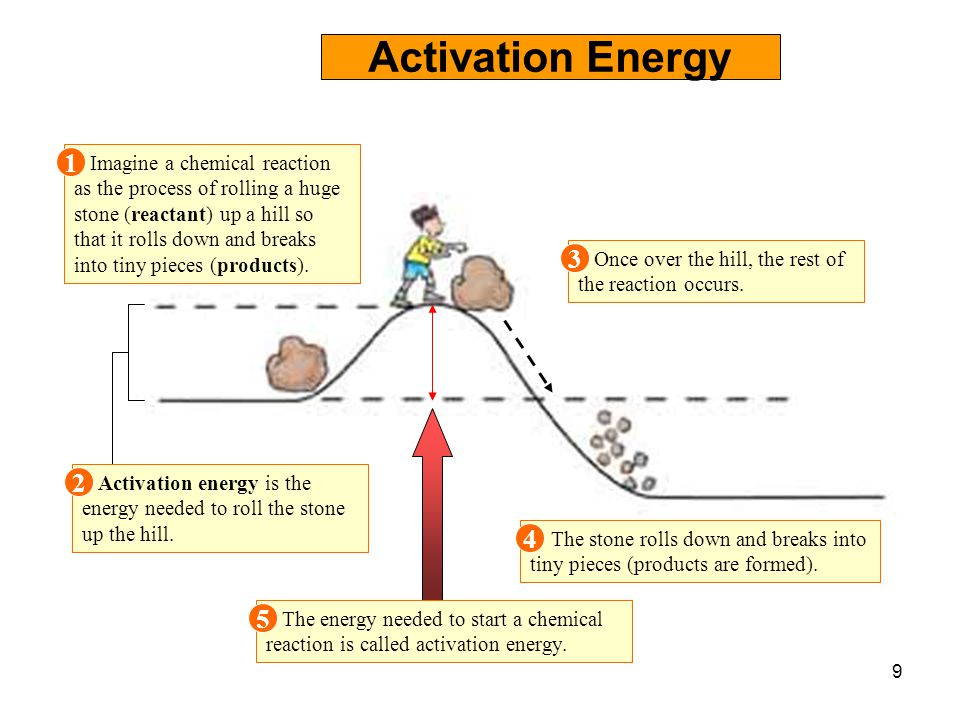
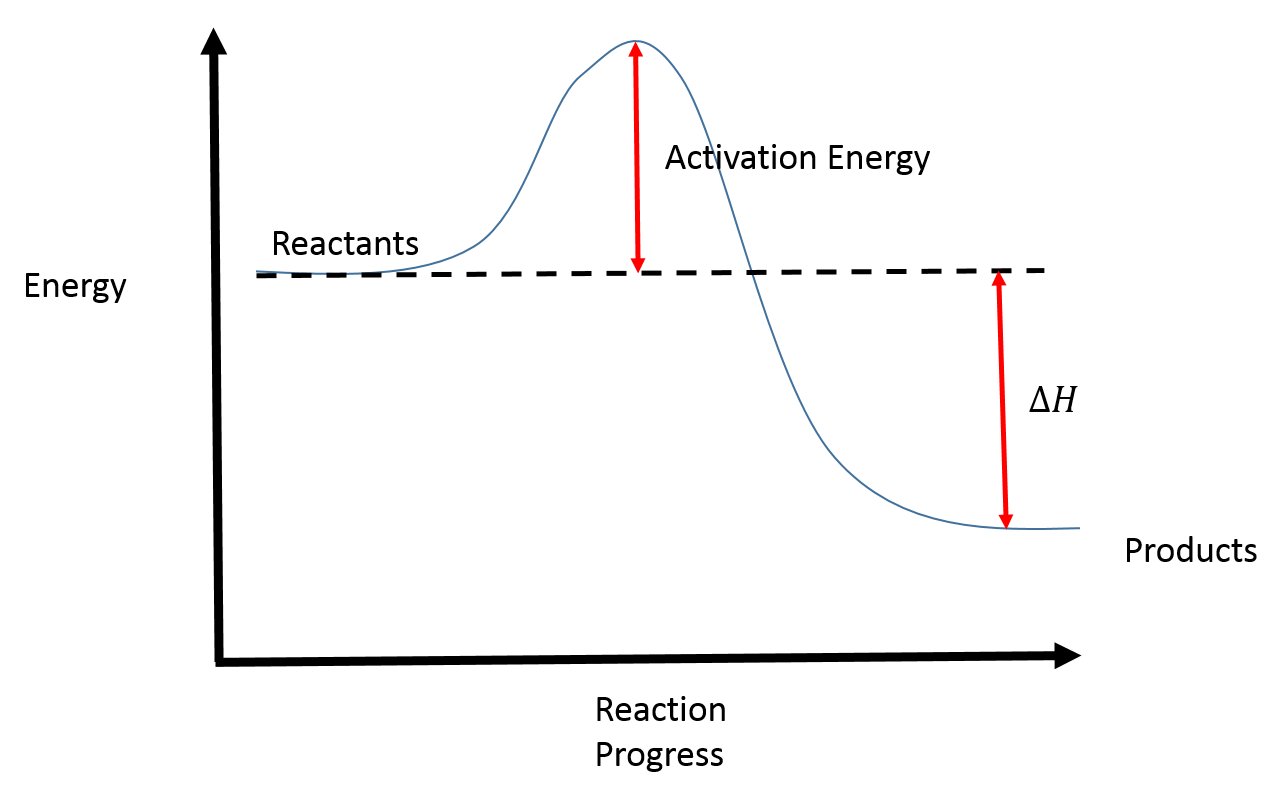
At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in. If the reaction were to proceed in the reverse direction (endergonic), the transition state would remain the same, but the activation energy would be. Web the activation energy is present in this graph but describes the amount of energy which reactant particles must have to break their bonds in the transition state. If the initial state has a lower potential energy than the. The higher the energy hill, the slower the reaction. Web in a diagram, activation energy is graphed as the height of an energy barrier between two minimum points of potential energy. Web the activation energy is what determines the kinetics of a reaction:
314 (Triple only) draw and explain reaction profile diagrams showing
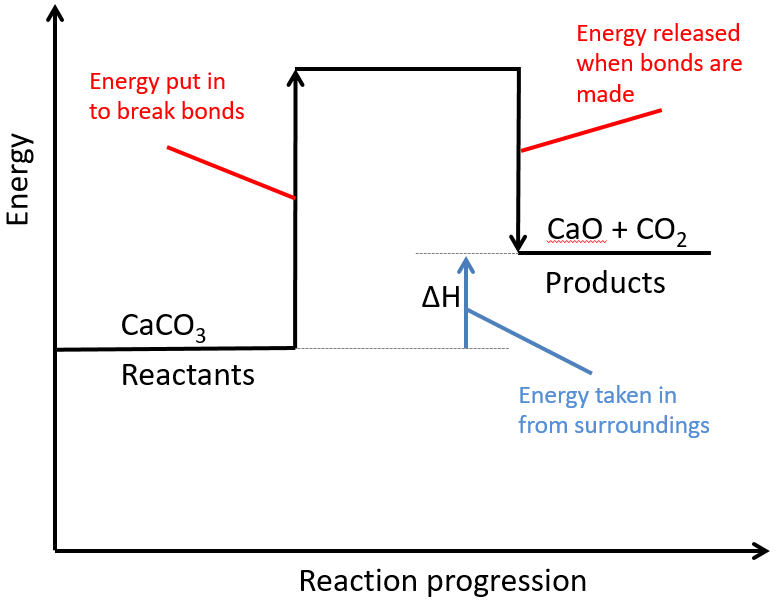
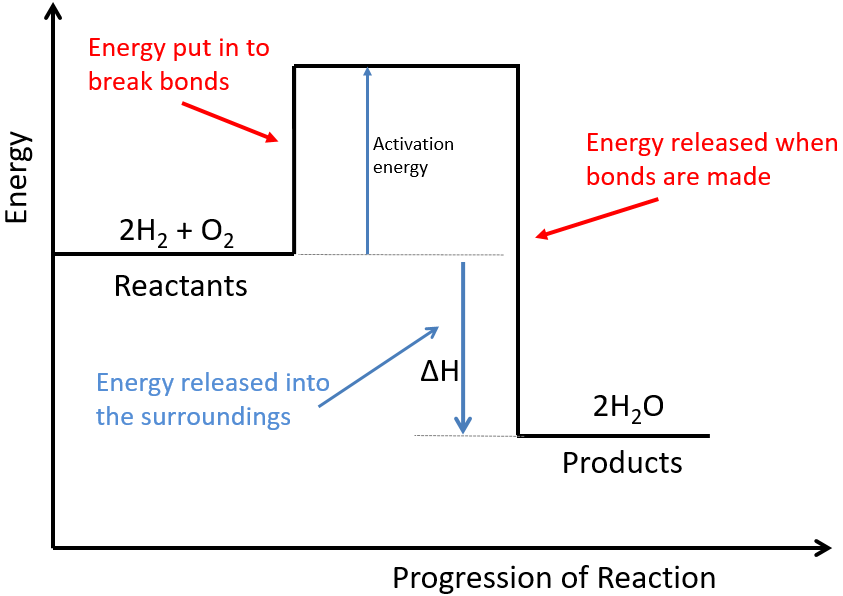
The activated complex is an unstable, intermediate product that is formed during the reaction. State one reason, in terms of energy, to support your answer. Web the energy difference between reactants and the transition state.
Activation Energy Definition, Formula, SI Units, Examples, Calculation
Web exothermic energy diagram: Web drawing reaction profiles reaction profiles show relative energies. Web solution we can obtain the activation energy by plotting ln k versus , knowing that the slope will be equal to..
Enzymes Lower The Activation Energy Of A Reaction btccasting
Draw the energy level diagram. Web the activation energy for a reaction is the minimum energy that colliding particles must have in order to undergo a reaction. The higher the energy hill, the slower the.
314 (Triple only) draw and explain reaction profile diagrams showing
Web the activation energy is what determines the kinetics of a reaction: An enzyme or catalyst lowers the activation energy. Web the energy difference between reactants and the transition state is called the activation energy,.
What are activation energies? Socratic
Draw and label a pair of axes. Web the activation energy is what determines the kinetics of a reaction: The higher the energy hill, the slower the reaction. The minimum points are the energies of.
Activation Energy The Secret to Getting Started and Getting Finished
At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in. In this video, i go over how to properly.
Activation Energy The Secret to Getting Started and Getting Finished
There must be a hump in the curve to represent the energy level of the activated complex. First determine the values of ln k and , and plot them in a graph: The activated complex.
Energy Diagram — Overview & Parts Expii
(jerry crimson mann, cc 3.0) effect of enzymes and catalysts a catalyst lowers the activation energy of a chemical reaction. E a = activation energy. The higher the energy hill, the slower the reaction. The.
Reaction Coordinate Diagrams College Chemistry
The higher the energy hill, the slower the reaction. Draw and label two short horizontal lines to mark the energies of the reactants and products. Web the activation energy shown in the diagram below is.
Activation energy vector illustration example diagram Physics and
The activation energy (e a) of a reaction is measured in kilojoules per mole (kj/mol) or kilocalories per mole (kcal/mol). The energy profile can also be used to determine the overall change in energy for.
Activation Energy Drawing Web the activation energy is what determines the kinetics of a reaction: Even exothermic reactions, such as burning a candle, require energy input. The activated complex is an unstable, intermediate product that is formed during the reaction. Web activation energy is indicated by the symbol e a and has units of joules (j), kilojoules per mole (kj/mol), or kilocalories per mole (kcal/mol). If the reaction were to proceed in the reverse direction (endergonic), the transition state would remain the same, but the activation energy would be.