Draw A Successive Ionization Energy Diagram For Aluminum
Draw A Successive Ionization Energy Diagram For Aluminum - For example, for p, the 5th ie is 6,270, while the 6th ie is 21,200. Thus, many students find it confusing that, for example, the 5p orbitals fill immediately after the 4d, and immediately before the 6s.the filling order is based on observed experimental results, and has been confirmed by theoretical calculations. Web so without actually providing the ionization energies for all the group 13 elements, they could say that the element has the second highest first ionization energy in its group, which would be aluminum. Web ionization energy increases here. In this article, i have discussed in detail how to easily write the complete electron configuration of aluminum.
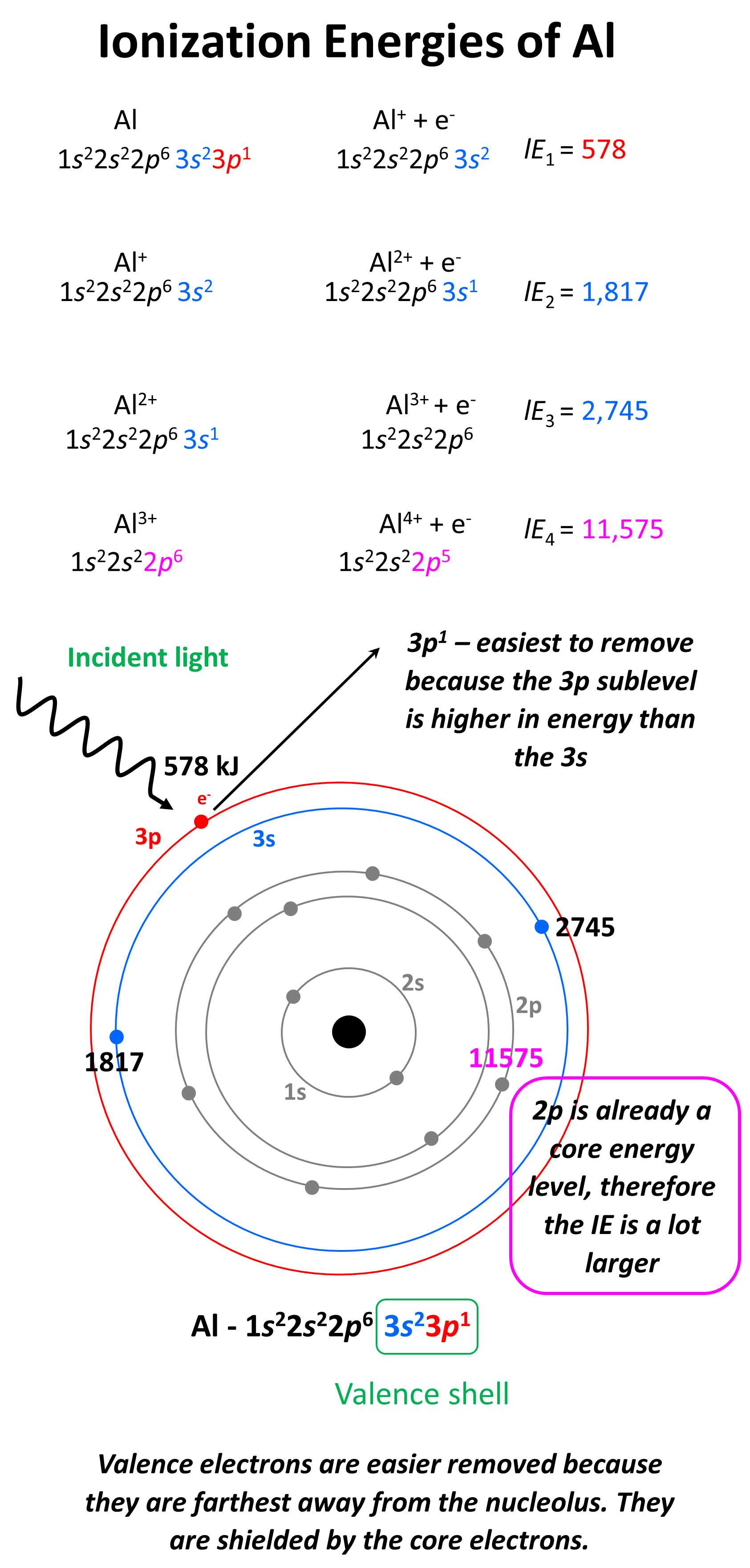
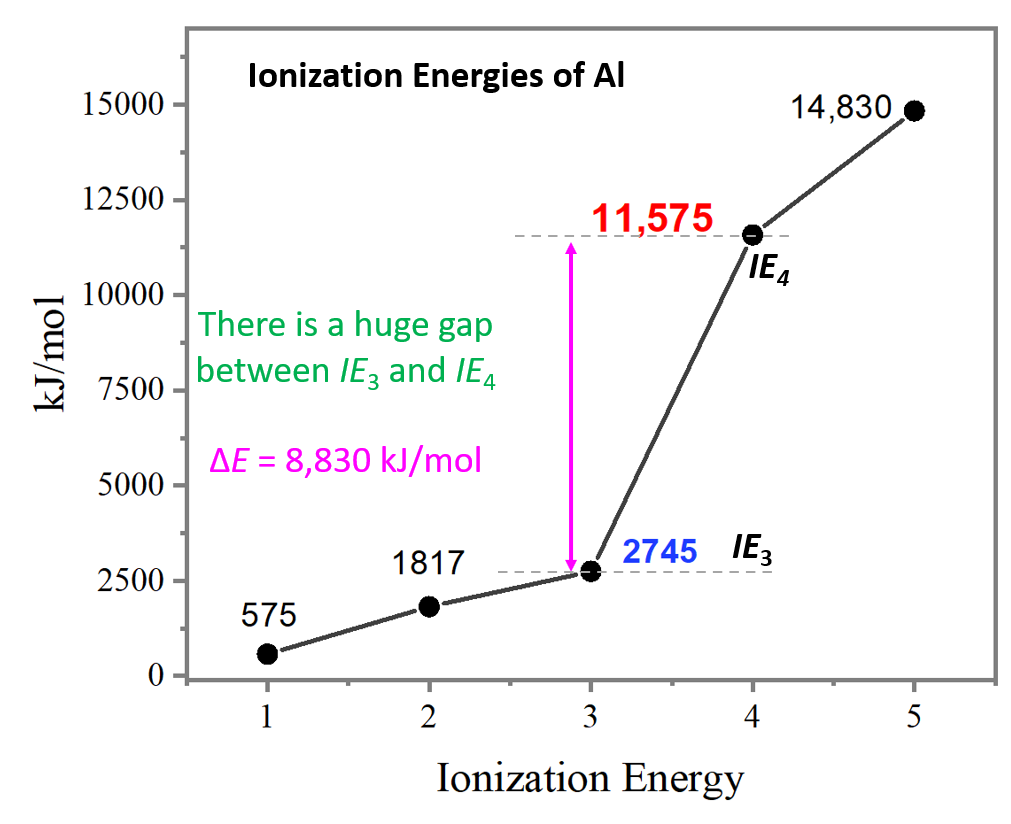
The ionization energy is measured in joules (j) or electron volts (ev). Web al,z = 13:1s22s22p63s23p1. But even that wouldn’t work well since gallium (the element beneath aluminum) has about the same first ionization energy as aluminum. This jump corresponds to removal of the core electrons, which are harder to remove than the valence electrons. It would start off with the lowest ionization energy. As you go from left to right, you go from low ionization energy to high ionization energy. For example, for p, the 5th ie is 6,270, while the 6th ie is 21,200.
Atomic structure
On the periodic table, first ionization energy generally increases as you move left to right across a period. The first ionization energy is the energy required to remove the outermost (valence). 3rd ionization energy, 2881.
Ionization energy Chemistry Steps
Web so, this is high, high ionization energy, and that's the general trend across the periodic table. It would start off with the lowest ionization energy. The ionization energy is measured in joules (j) or.
Ionisation Energy AS Level Teaching Resources
Web in a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make covalent or ionic bonds with each other. Web the successive ionization energy diagram is.
Question Video Correlation between Ionization Energy and Electron
Web al,z = 13:1s22s22p63s23p1. Web in a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make covalent or ionic bonds with each other. 4th ionization energy,.
Ionization energy Chemistry Steps
1s 2 2s 2 2p 6 3s 2: Web successive ionization energies remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral atom.
12.1 Successive ionisation energies (HL) YouTube
In this article, i have discussed in detail how to easily write the complete electron configuration of aluminum. I 1 i_1 i 1 = 578 kj/mol. That is because aluminum has three valence electrons that.
Explaining Successive Ionisation Energies YouTube
Web electron configuration for aluminum (al, al3+ ion) aluminum is the 13th element in the periodic table and its symbol is ‘al’. 2nd ionization energy, 1816 kj ⋅ mol−1; I 4 i_4 i 4 =.
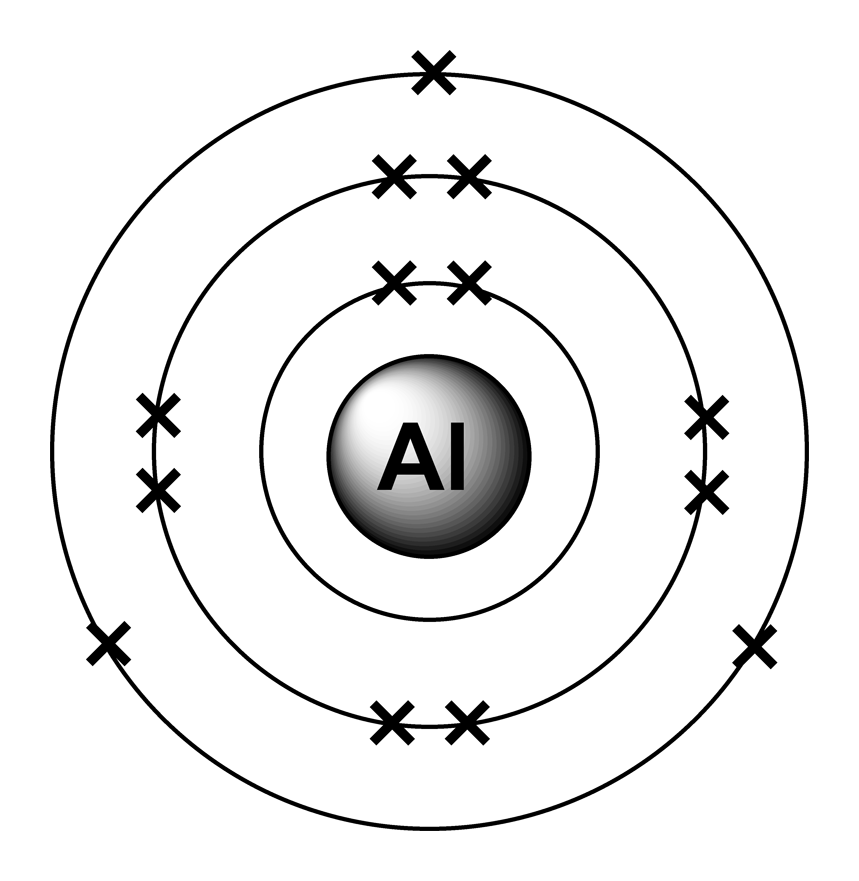
Electron arrangements
The ionization energy that corresponds to removing an electron from the noble gas configuration would be substantially higher than those before. First ionization energy, second ionization energy as well as third ionization energy of the.
Atomic structure
The values mentioned in the chart are given in electron volts (ev). On the periodic table, first ionization energy generally increases as you move left to right across a period. 2nd ionization energy, 1816 kj.
Successive Ionisation Energy vigglegiggle
Web label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) on diagram above. In this article, i have discussed in detail how to easily write the complete electron.
Draw A Successive Ionization Energy Diagram For Aluminum Web ionization energy chart of all the elements is given below. Now, what about trends up and down the periodic table? Web al,z = 13:1s22s22p63s23p1. An element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. Web ionization energy increases here.