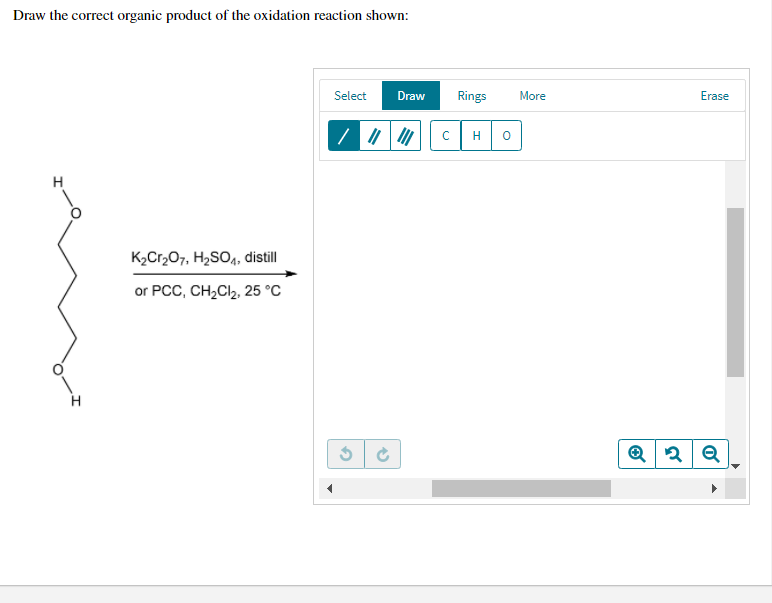
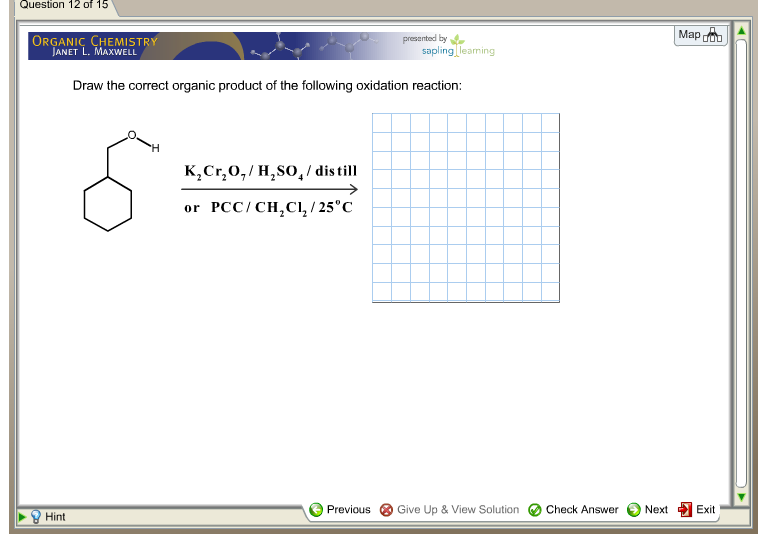
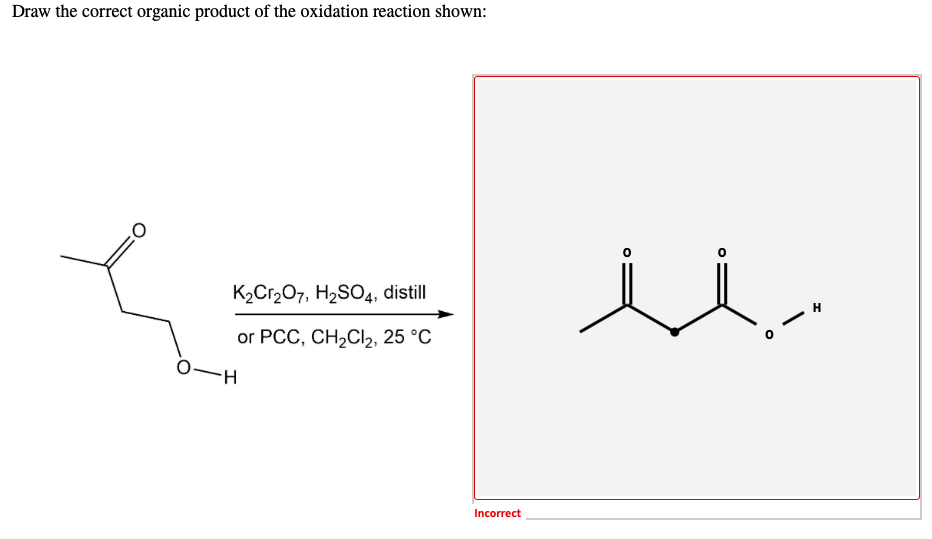
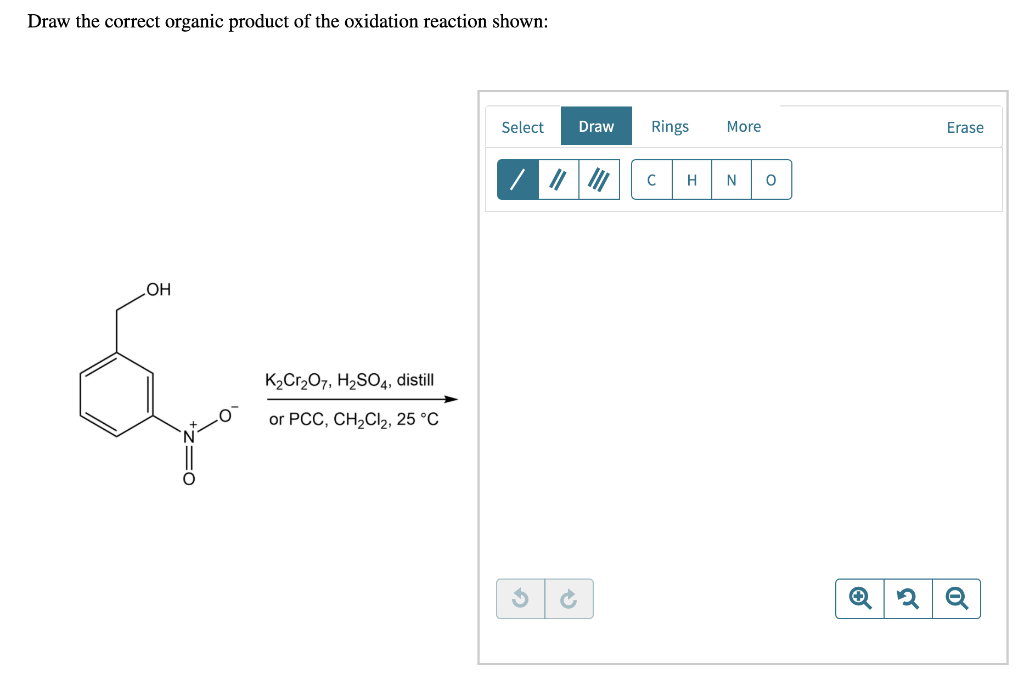
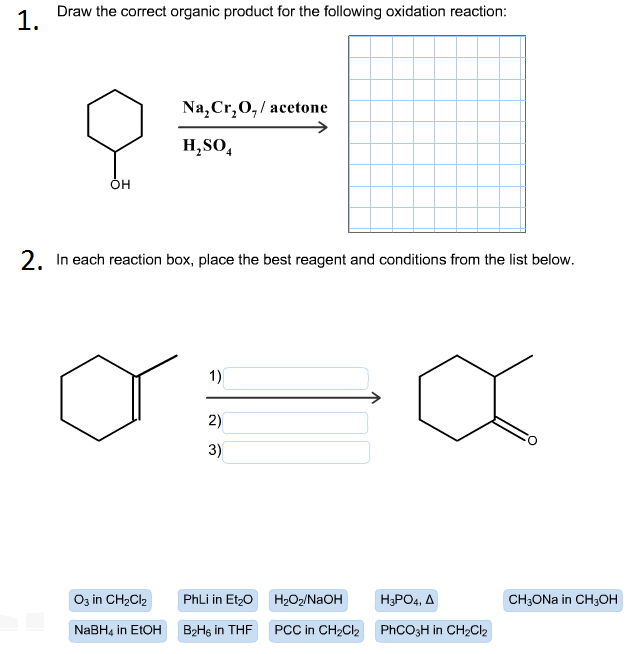
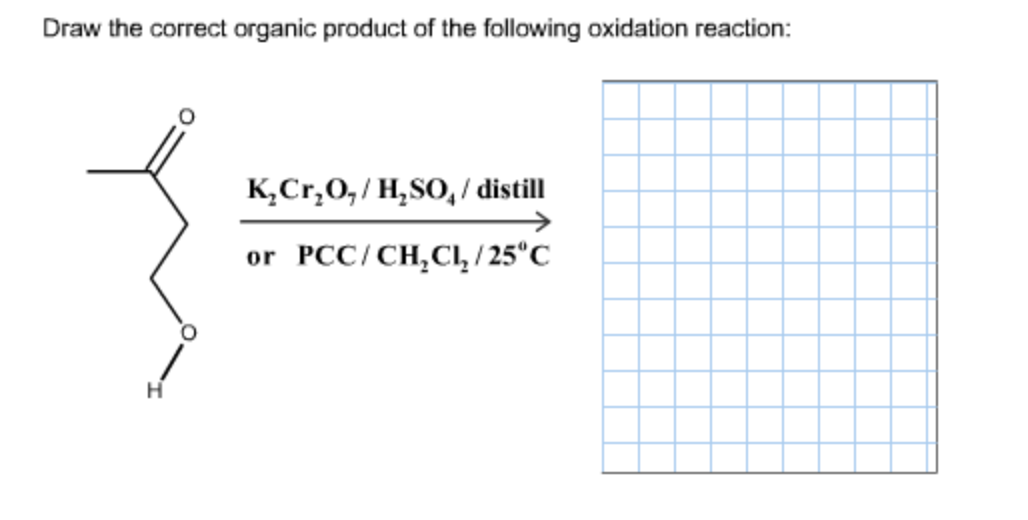
Draw The Correct Organic Product For The Oxidation Reaction
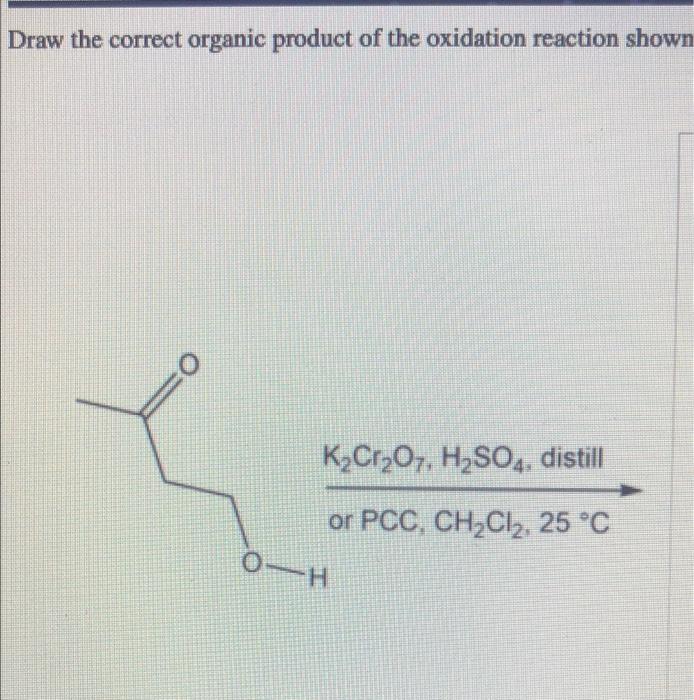
Draw The Correct Organic Product For The Oxidation Reaction - This reduced compound is also called the oxidizing agent. Web jay is looking at one of the reactants and comparing it to one of the products to see whether or not carbon underwent oxidation or reduction. H k2cr2o7, h2so4, distill or pcc, ch2cl2, 25 °c this problem has been solved! The byproducts (featured in grey) are cr (iv) as well as pyridinium hydrochloride. If it converts a compound to a lower oxidation level it is a reduction.
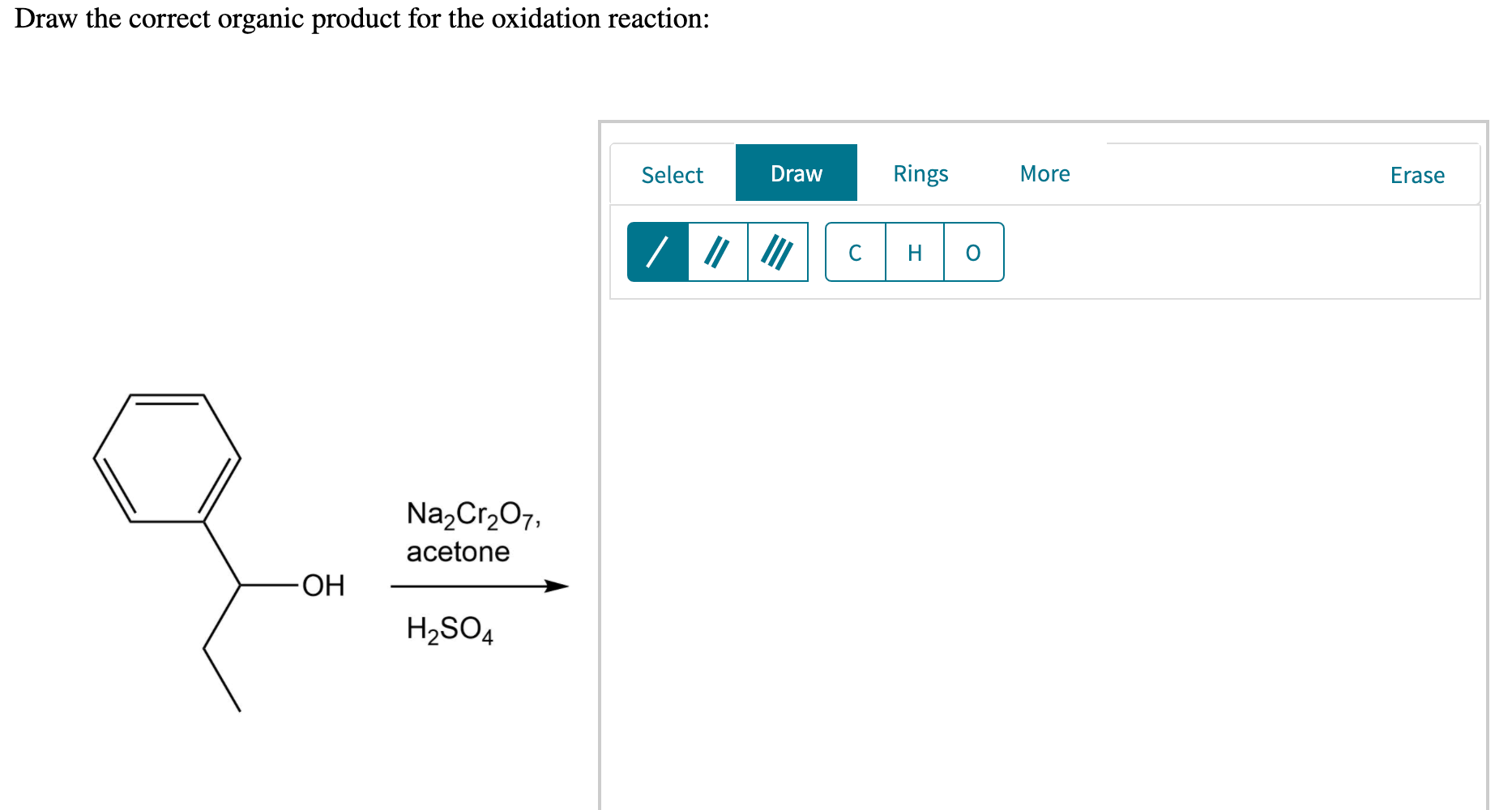
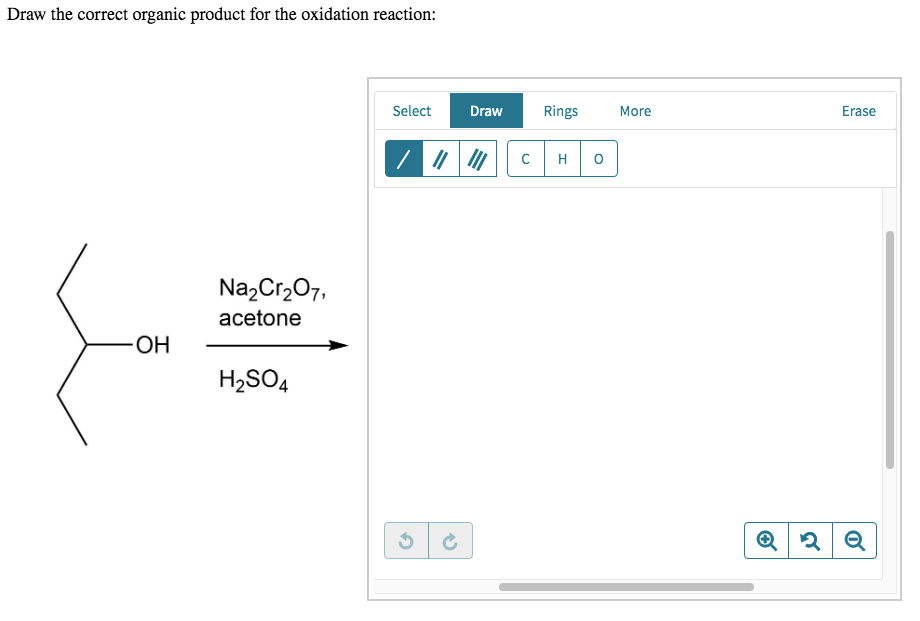
For example, chromium trioxide (cro 3) is a common oxidizing agent used by organic chemists to oxidize a secondary alcohol to a ketone. Web for an alcohol to be oxidized in a reaction there must also be a compound being reduced. If you add one equivalent of pcc to either of these alcohols, you obtain the oxidized version. Two examples of organic redox reactions are shown below. Draw the correct product for the reaction. If the oxidation level of the reactant does not change it is not a redox reaction. Draw the correct organic product for the oxidation reaction:
Solved Draw the correct organic product for the oxidation
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web jay is looking at one of the reactants and comparing it to one of the products to see.
Solved Draw the correct organic product for the oxidation
Web here are two examples of pcc in action. Web draw the correct organic product of the oxidation reaction shown: If the oxidation level of the reactant does not change it is not a redox.
Solved Draw the correct organic product of the oxidation
H k2cr2o7, h2so4, distill or pcc, ch2cl2, 25 °c this problem has been solved! Web organic chemistry with a biological emphasis (soderberg) 15: Web if a reaction converts a compound to a higher oxidation level.
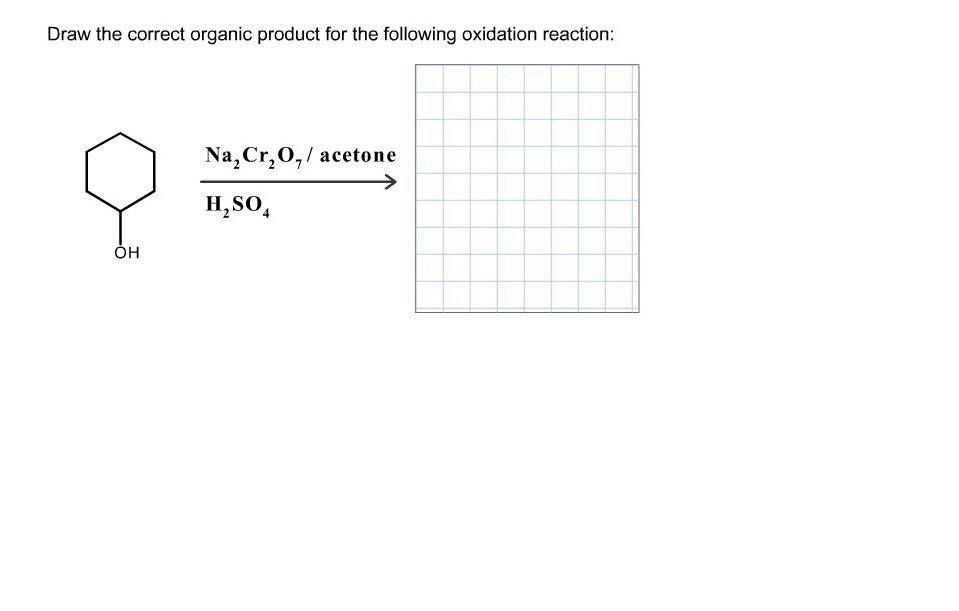
Solved Draw the correct organic product for the following
One has to be careful with the amount of water present in the reaction. Two examples of organic redox reactions are shown below. To identify the oxidizing agents and the reducing agents we have to.
Solved Draw the correct organic product of the oxidation
If it converts a compound to a lower oxidation level it is a reduction. H k2cr2o7, h2so4, distill or pcc, ch2cl2, 25 °c this problem has been solved! Web jay is looking at one of.
Solved Draw the correct organic product of the following
If it converts a compound to a lower oxidation level it is a reduction. During this reaction cro 3 is being reduced to form h 2 cro 3. You'll get a detailed solution from a.
Solved Draw the correct organic product of the oxidation
H k2cr2o7, h2so4, distill or pcc, ch2cl2, 25 °c this problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This reduced compound is also.
Solved Draw the correct organic product of the oxidation
This reduced compound is also called the oxidizing agent. Draw the correct organic product for the oxidation reaction: If the oxidation level of the reactant does not change it is not a redox reaction. Web.
Solved Draw the correct organic product for the following
This reduced compound is also called the oxidizing agent. Web here are two examples of pcc in action. The byproducts (featured in grey) are cr (iv) as well as pyridinium hydrochloride. You'll get a detailed.
Solved Draw The Correct Organic Product Of The Following
Web draw the major organic product for the reaction shown. If the oxidation level of the reactant does not change it is not a redox reaction. Web organic chemistry with a biological emphasis (soderberg) 15:.
Draw The Correct Organic Product For The Oxidation Reaction Draw the correct organic product for the oxidation reaction: To identify the oxidizing agents and the reducing agents we have to examine the entire reaction and identify which reactant species have atoms which are oxidized and which atoms get reduced. This reduced compound is also called the oxidizing agent. Web jay is looking at one of the reactants and comparing it to one of the products to see whether or not carbon underwent oxidation or reduction. One has to be careful with the amount of water present in the reaction.