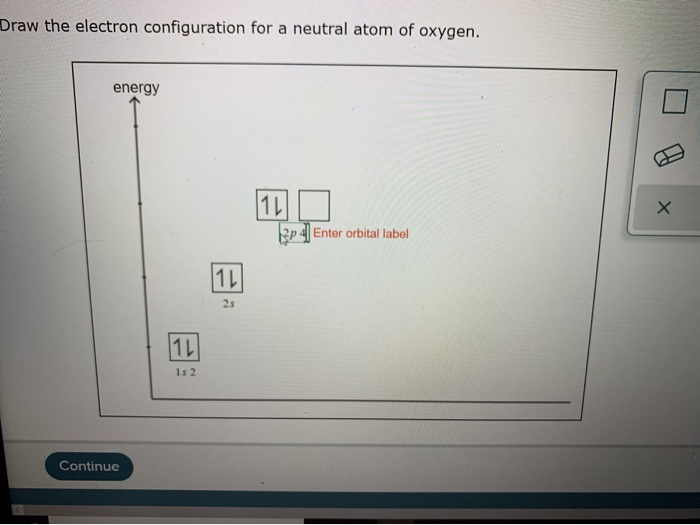
Draw The Electron Configuration For A Neutral Atom Of Oxygen
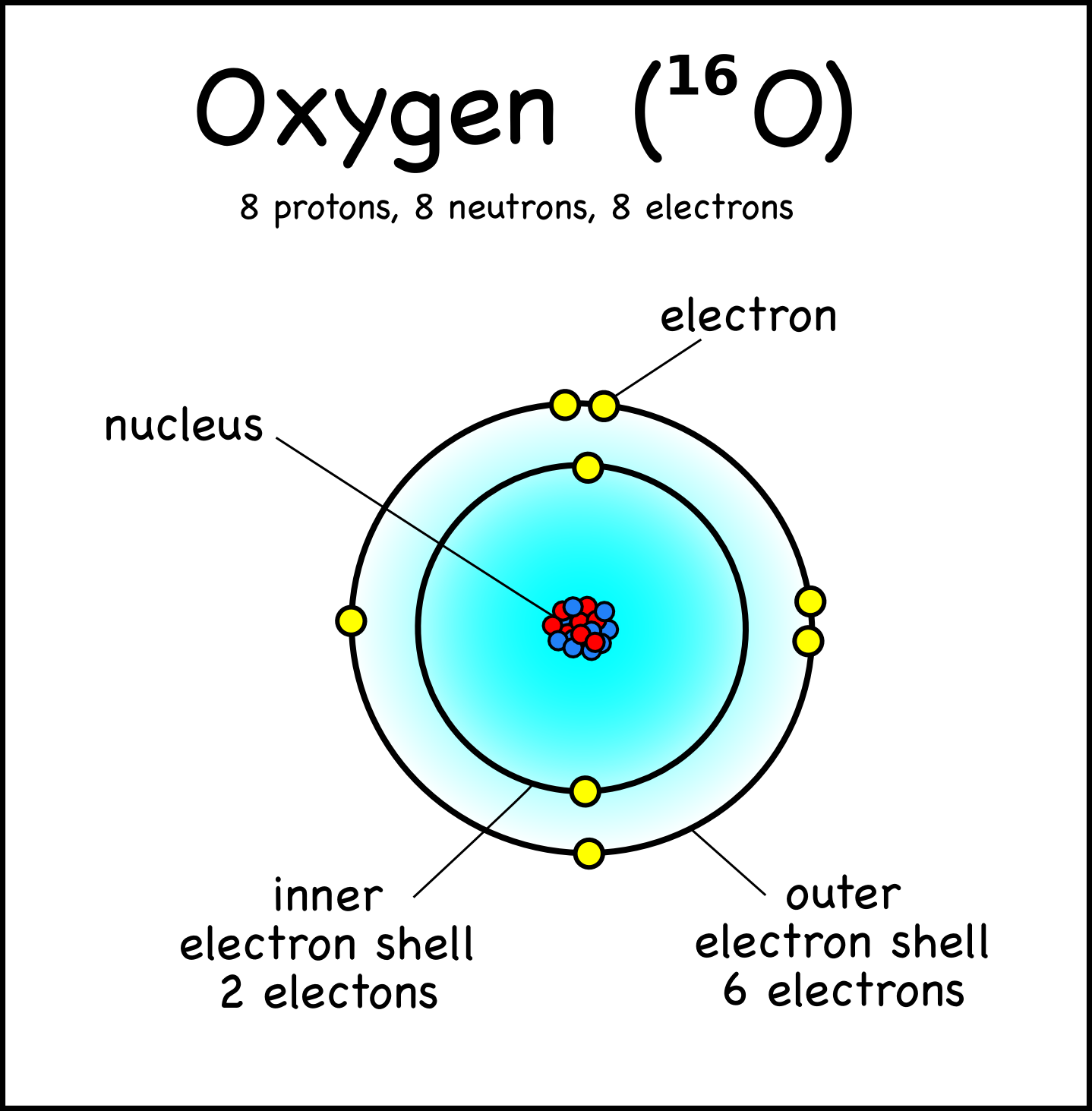
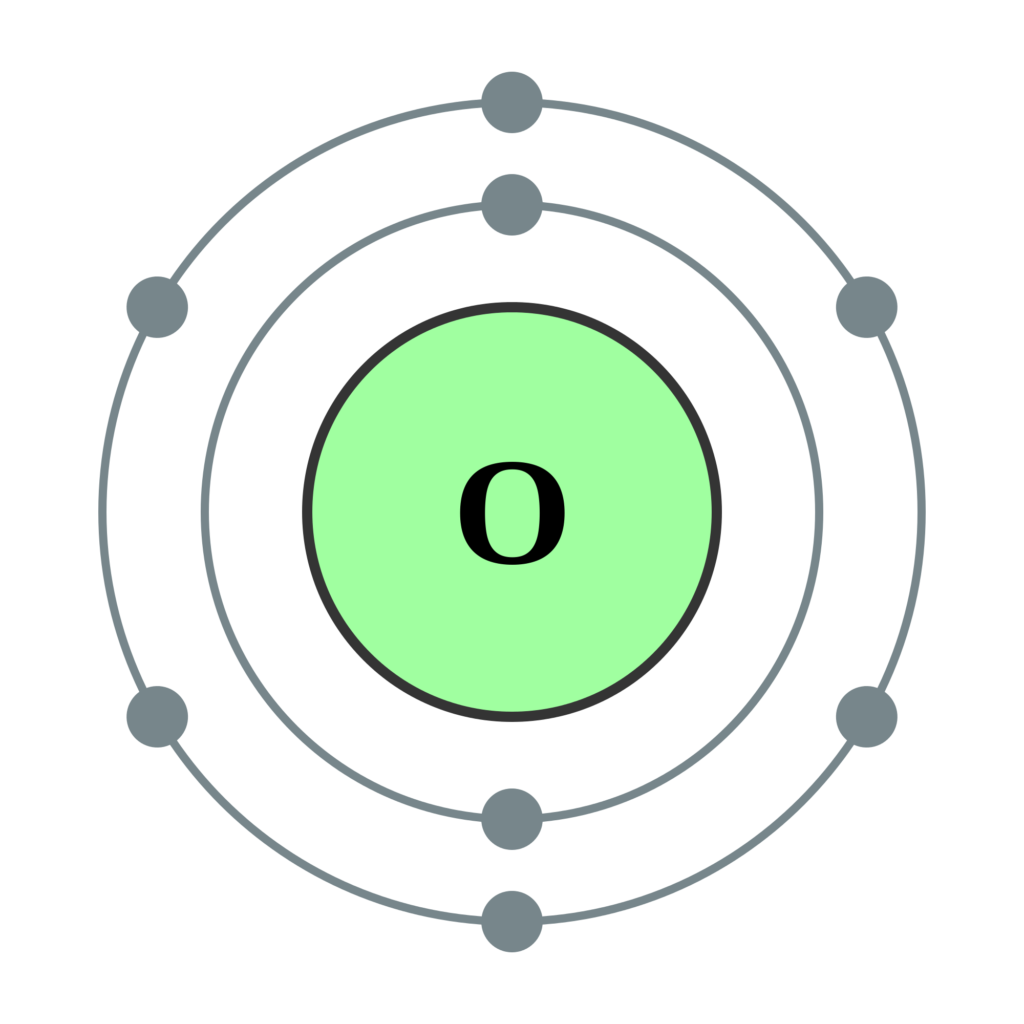
Draw The Electron Configuration For A Neutral Atom Of Oxygen - This means the first shell (1s) has 2. Web an oxygen atom is a neutral atom that has 8 atomic numbers which imply it has a total of 8 electrons. Si 4 + was formed by the loss of four electrons. Web because chemists are really interested in keeping track of where all the electrons in a given atom live, they write down a series of symbols called an electron configuration that keeps track of all of this information for a given atom. 1s 2 2s 2 2p 6 3s 1.
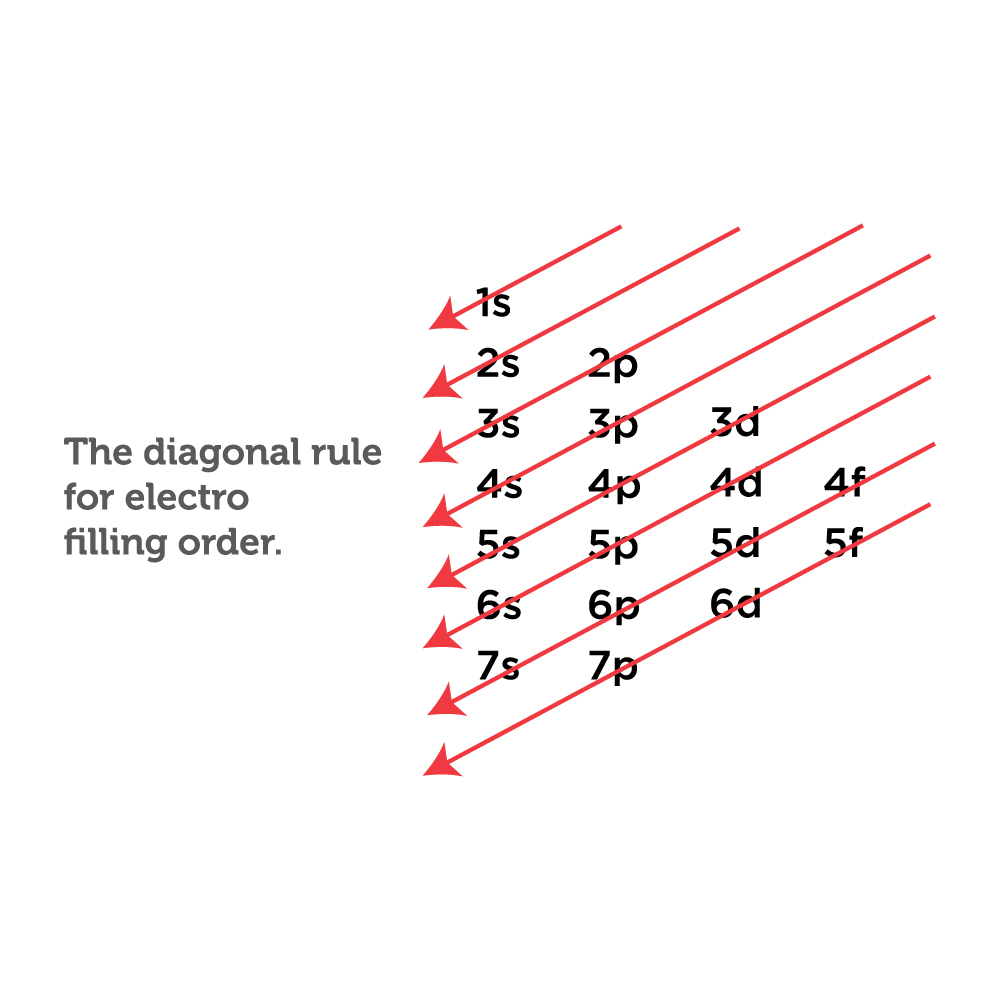
Web oxygen is the eighth element with a total of 8 electrons. For example, the electron configuration of oxygen looks like: Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. The remaining four electrons will go in the 2p orbital. 1s 2 2s 2 2p 2: Electron configuration of nitrogen (n) [he] 2s 2 2p 3: How do you draw the shell diagram of sodium atom?
Electron Configuration Chart With Orbitals
1s 2 2s 2 2p 6 3s 2 3p 2. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals.
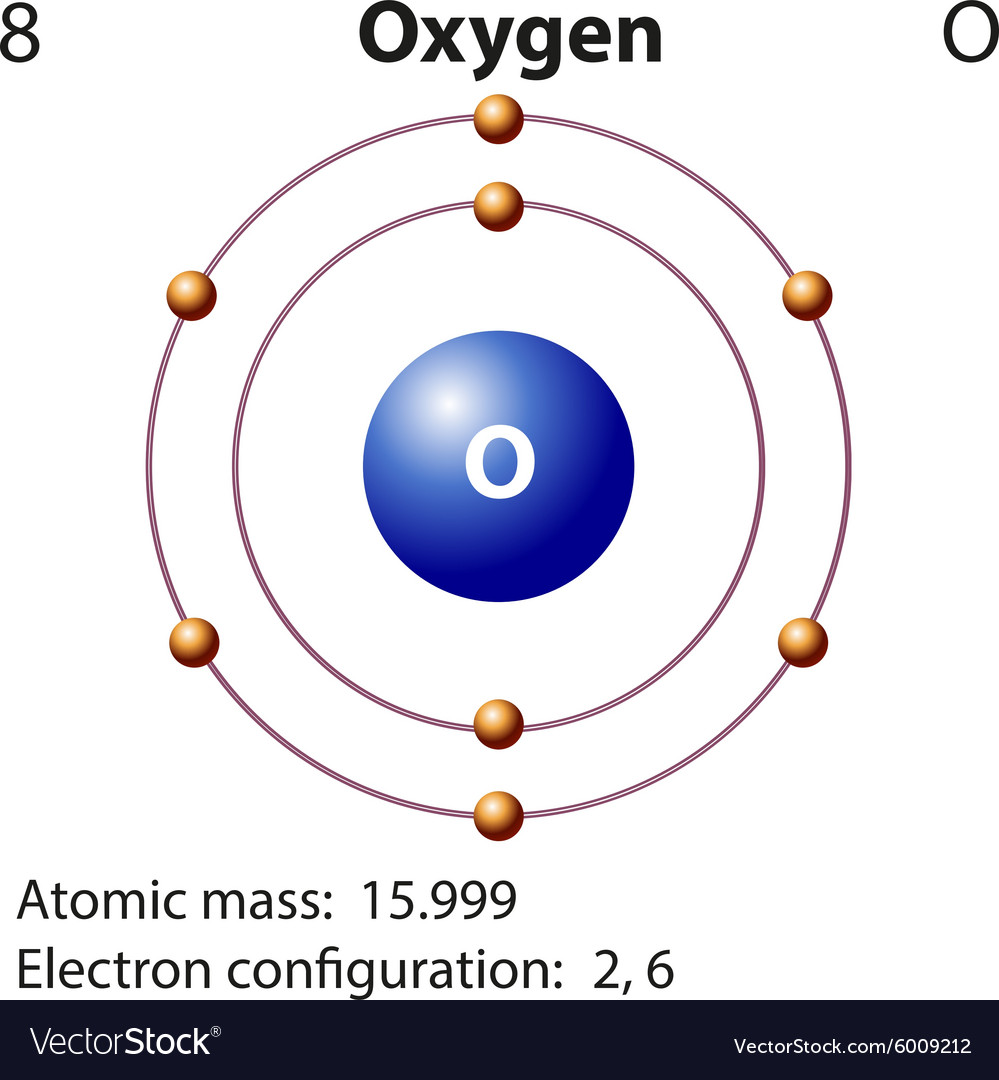
Diagram representation element oxygen Royalty Free Vector
What is the name of this atom? Draw lewis structures depicting the bonding in simple molecules; Web oxygen is the eighth element with a total of 8 electrons. Draw a lewis structure, showing all bonding.
Forms of Energy ND Studies Energy Curriculum
How many core electrons are there? Electron configuration of oxygen (o) [he] 2s 2 2p 4: Web elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). For example, oxygen has six valence.
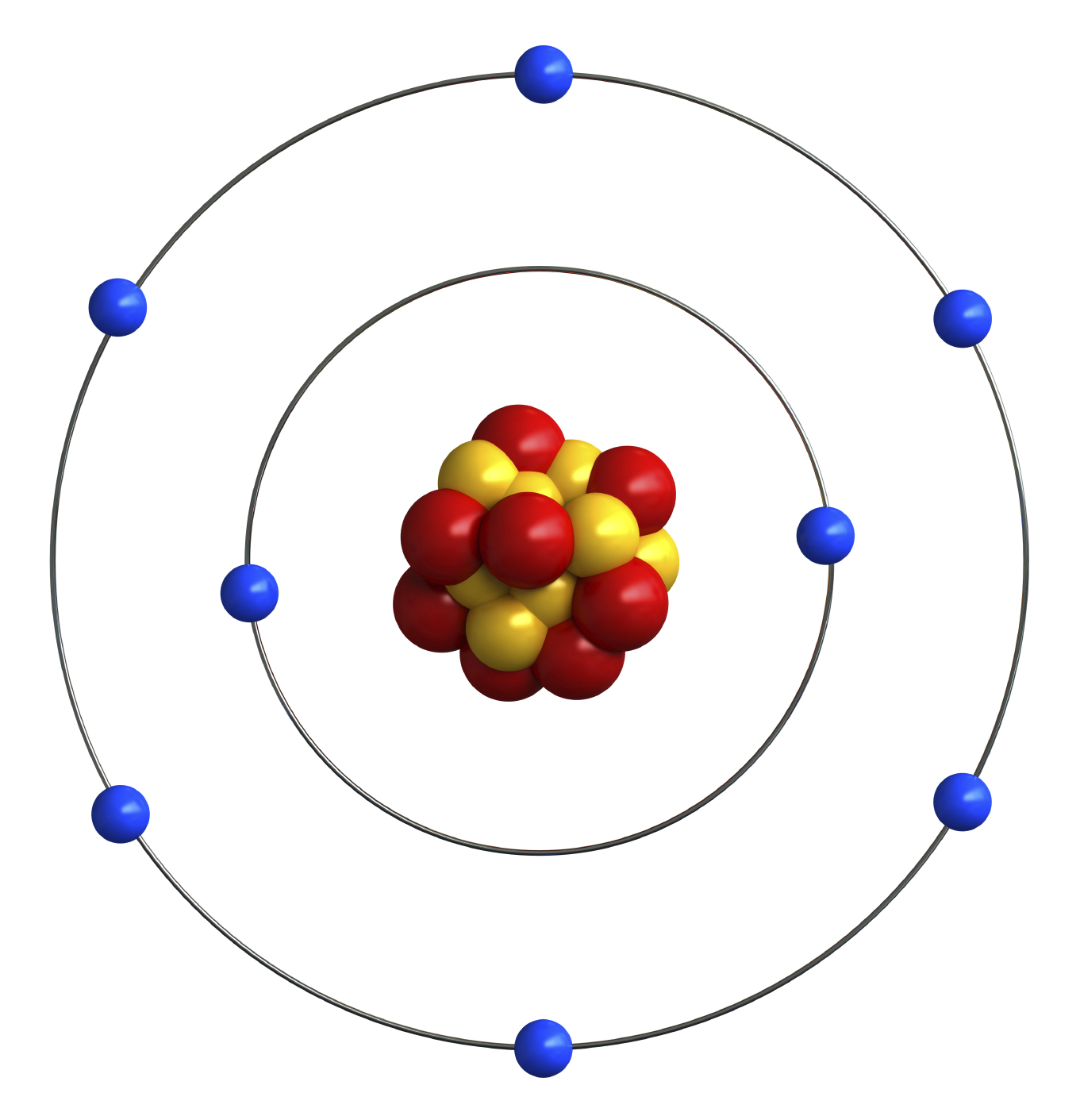
Bohr Model Oxygen Chemical Element Atomic Number, PNG, 1000x1000px
In order to write the o electron configuration we first need to know t. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital..
Oxygen Electron Configuration (O) with Orbital Diagram
Write the configuration of the neutral atom. From the pauli exclusion principle, we know that an orbital can contain two electrons with. And valence electrons are those electrons found in the outer shell of an.
Drawing Atoms Montessori Muddle
Electron configuration can be done in two ways. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding.
How to Write Ground State Electron Configuration in Chemistry
For example, the electron configuration of oxygen looks like: Oxygen has one more electron than nitrogen and as the orbitals are all half filled the electron must pair up. 1s 2 2s 2 2p 1:.
Solved Draw the electron configuration for a neutral atom of
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. 1s 2 2s 2 2p 6 3s 1. At oxygen, with z = 8 and eight electrons, we have no.
What is the Electron Configuration of Oxygen Archives Dynamic
However, it's easy to determine the configuration of electrons for heavier elements by making a chart. From the pauli exclusion principle, we know that an orbital can contain two electrons with. This means the first.
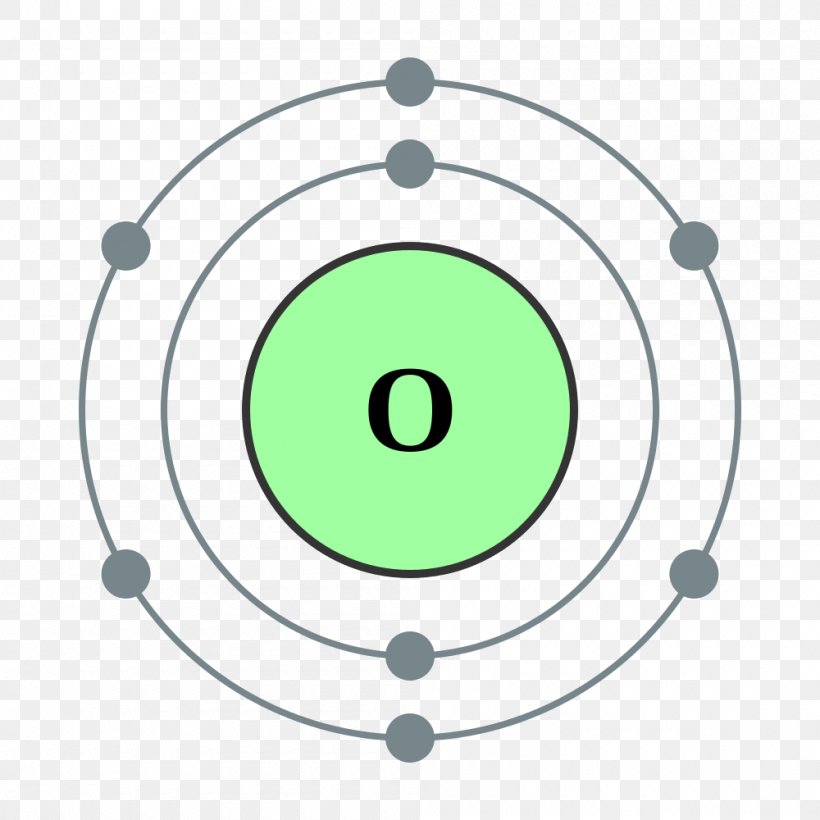
Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
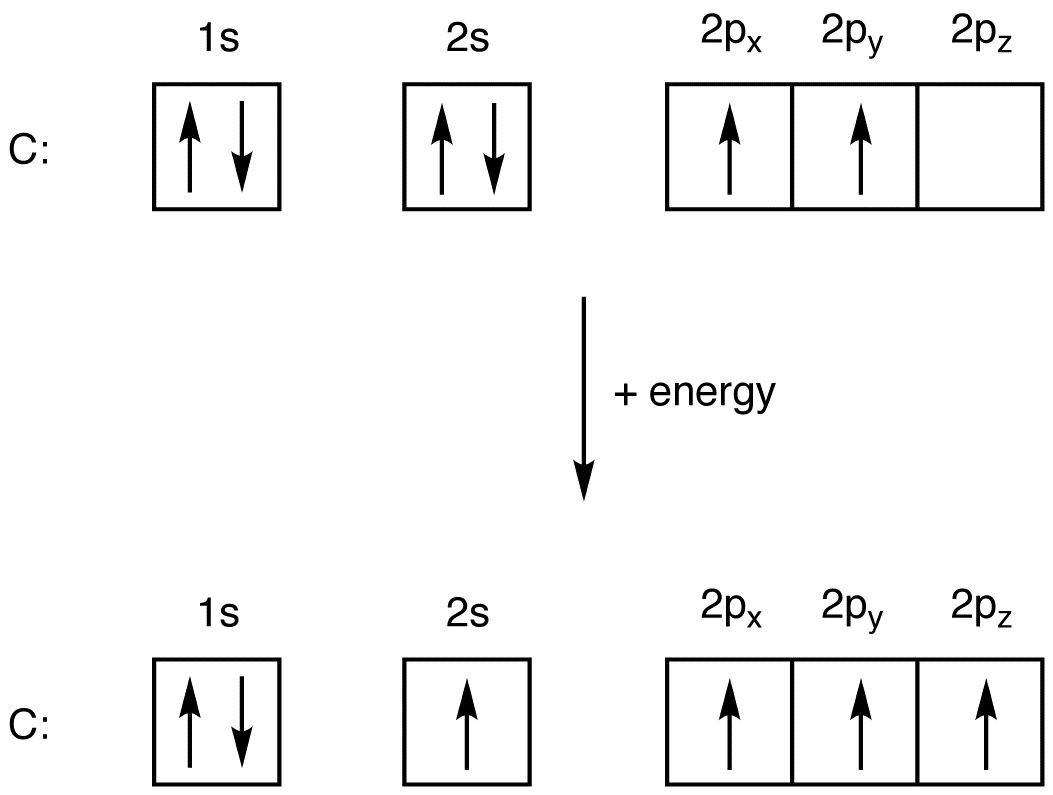
Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. The electron configuration of nitrogen is thus 1 s2 2 s2 2 p3. Electron configuration of carbon (c) [he] 2s.
Draw The Electron Configuration For A Neutral Atom Of Oxygen How many core electrons are there? Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. 1s 2 2s 2 2p 4: In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Write the configuration of the neutral atom.