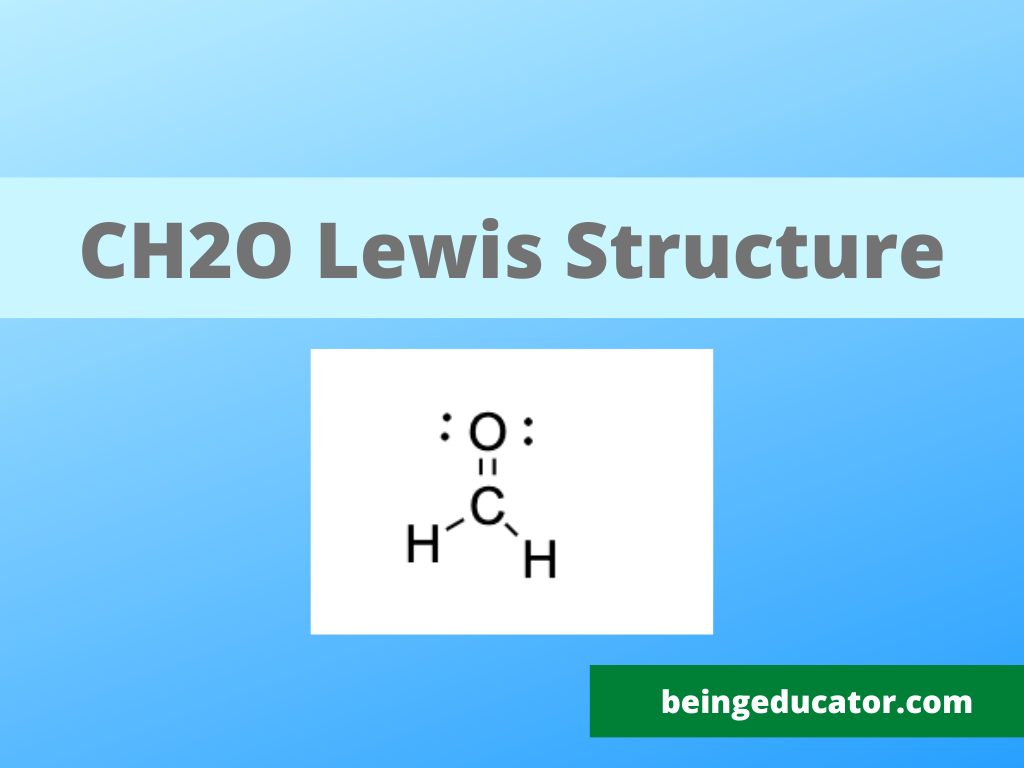
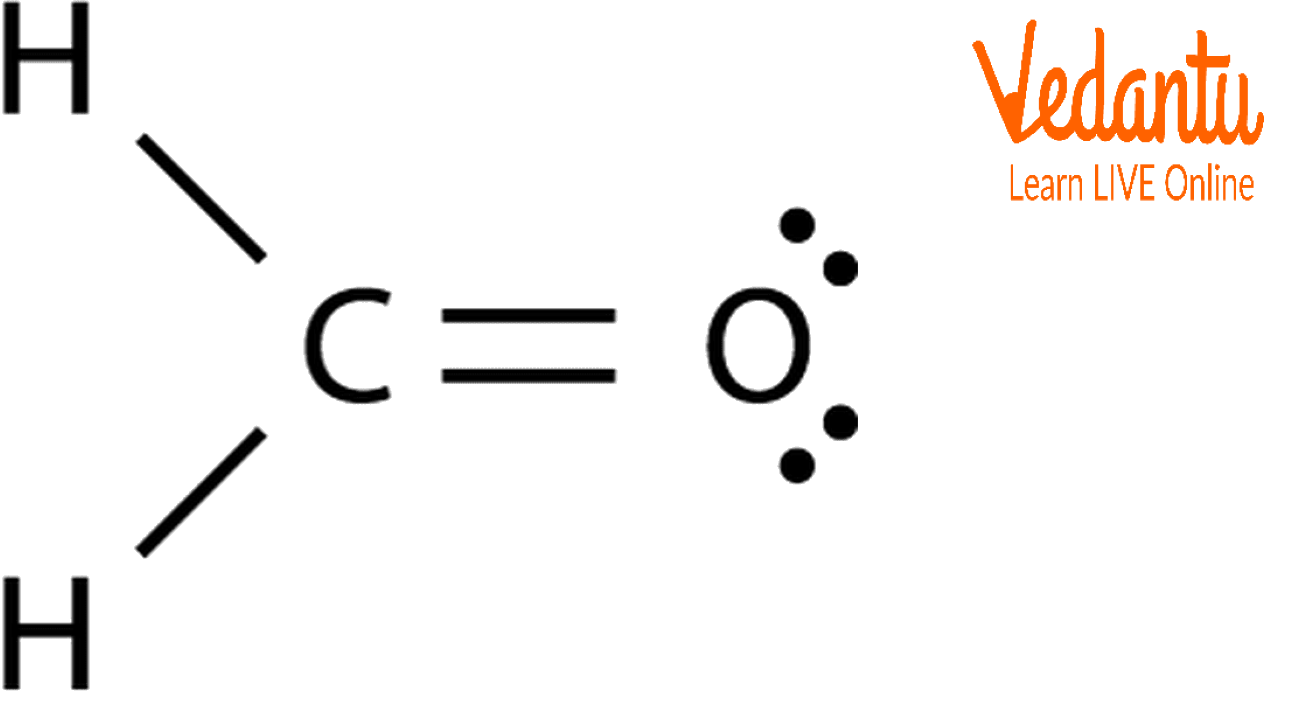Draw The Lewis Structure For Ch2O
Draw The Lewis Structure For Ch2O - The number of valence electrons for carbon is 4, oxygen is 6, and. What is the strongest intermolecular force this molecule wo exhibit? Search for how many more electrons are required to stabilize the octet of all the interacting atoms: Web chemistry chemistry questions and answers draw the best lewis structure for ch2o. Carbon (c) is the least electronegative atom in the ch2o lewis structure and therefore should be placed at the.
Web detailed guide on how to draw the lewis structure of ch2o, its molecular geometry, hybridization, polarity, and frequently asked questions on the topic. Based on the lewis structure for ch2o, consider the following. \(\ce{n2}\) \(\ce{ch2o}\) (the carbon atom is the central atom.) one application of ch 2 o, also called formaldehyde, is the preservation of biological specimens. In order to find the total valence electrons in ch2o molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. Identify the total number of valence electrons for each atom. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. For the ch 2 o lewis structure there are a total of 18 valence electrons available.
Draw The Lewis Structure For Co Molecule Ch2o Molecule Fotodtp
Select the center atom (h is always outside). If hydrogen is present in the molecule, always place the hydrogen atoms on the. While selecting the center atom, always put the least. Web = 4+6+2*1 =.
CH2O Molecular Geometry / Shape and Bond Angles YouTube
(1) find the number of valence electrons in the molecule. Web drawing lewis structures for molecules with one central atom: With two bonding pairs and two lone pairs, the oxygen atom has now completed its.
CH2O Lewis Structure Lewis Dot Structure for CH2O Methanal or
With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Web the lewis diagram of a molecule can be constructed using the following stepwise procedure: (2) draw single bonds.
ch2o lewis structure
Web i quickly take you through how to draw the lewis structure of ch2o (methanal or formaldehyde). Figure out how many electrons the molecule must have, based on the number of valence electrons in each.
CH2O Lewis Structure How to Draw the Dot Structure for CH2O YouTube
Web i quickly take you through how to draw the lewis structure of ch2o (methanal or formaldehyde). Here’s how you can easily draw the ch 2 o lewis structure step by step: Find the total.
How to Draw the Lewis Dot Structure for CH2O (Formaldehyde) YouTube
(2) draw single bonds between bonded atoms. Web learn the steps to draw the lewis structure of ch2o (formaldehyde) in just 1 minute. Formaldehyde lewis structure lewis structure is a pictorial representation of the atoms.
CH2O Lewis Structure, Molecular Geometry, and Hybridization
(3) distribute the remaining electrons throughout the molecule, keeping in mind the duet and octet rules. What is the strongest intermolecular force this molecule wo exhibit? It is a chemical formula for methanol or what.
CH2O Polar or Nonpolar Learn Important Terms and Concepts
Web = 4+6+2*1 = 12 valence electrons of ch2o thus, ch2o has a total of twelve valence electrons that can help in drawing its lewis structure. Find the total valence electrons in ch2o molecule. It.
CH2O Lewis Structure, Molecular Geometry, and Hybridization
Web the lewis structure of ch2o is drawn as: Calculate the total number of valence electrons. Search for the total already available valence electrons in a single formaldehyde ch2o molecule: Web the structure on the.
lewis structure of ch2o
Select the center atom (h is always outside). Web drawing the ch2o lewis structure involves several steps: Carbon (c) is the least electronegative atom in the ch2o lewis structure and therefore should be placed at.
Draw The Lewis Structure For Ch2O It is a chemical formula for methanol or what is also commonly referred to as formaldehyde. Web the lewis structure of ch2o is drawn as: Web = 4+6+2*1 = 12 valence electrons of ch2o thus, ch2o has a total of twelve valence electrons that can help in drawing its lewis structure. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. What is the strongest intermolecular force this molecule wo exhibit?