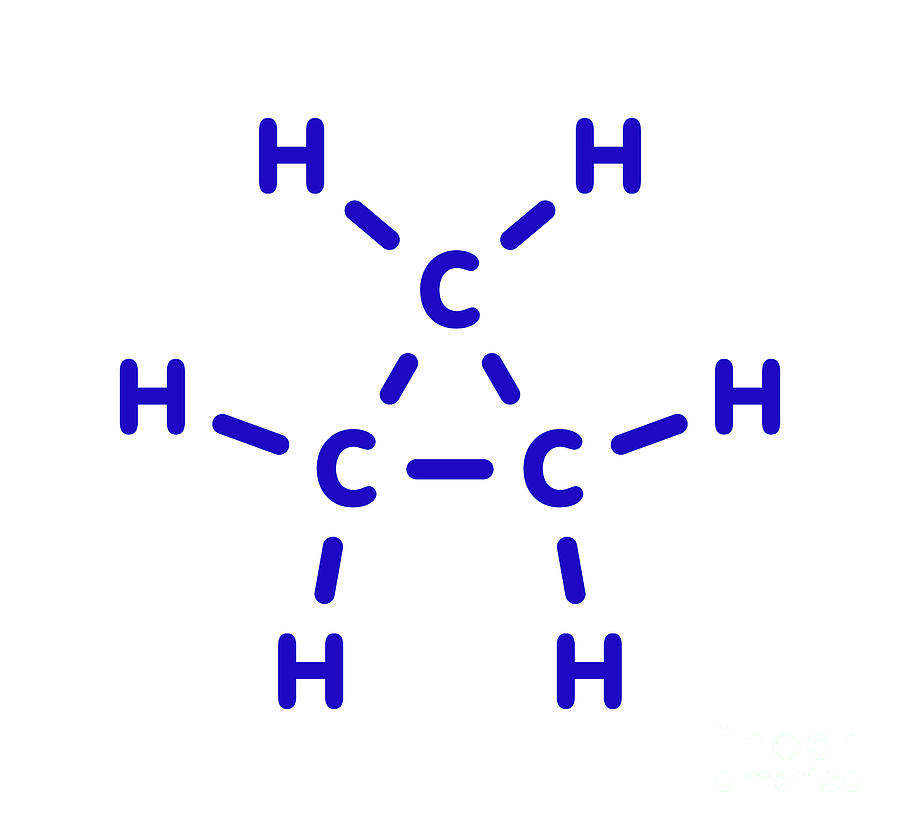
Draw The Structure Of Cyclopropane
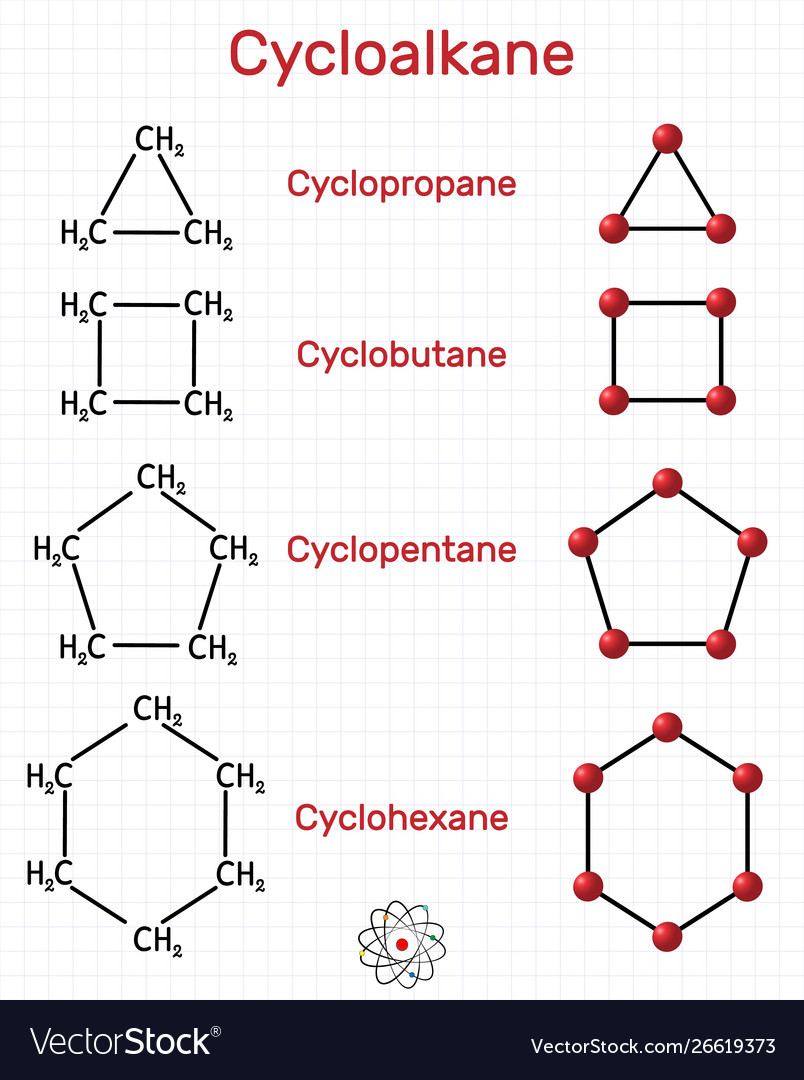
Draw The Structure Of Cyclopropane - Note the triangle between the three carbon atoms for cyclopropane, and the square between the four carbon atoms for cyclobutane. The molecule has d molecular symmetry. Web draw the structures and give the name of the 8 constitutional isomers withe the molecular formula c9h12 that contain a benzene ring. Web describe, and sketch the conformation of cyclopropane, cyclobutane, and cyclopentane. It can asphyxiate by the displacement of air and has a narcotic effect in high concentration (formerly used as an anesthetic gas).
The chemistry of alkyl halides Contact with the liquid may cause frostbite. Figure 4.5 the structure of cyclopropane, showing the eclipsing of neighboring c. Web draw the structure of cyclopropane this problem has been solved! Web describe, and sketch the conformation of cyclopropane, cyclobutane, and cyclopentane. In addition, cyclopropane has considerable torsional strain because the c−h bonds on neighboring carbon atoms are eclipsed ( figure 4.5 ). It can be represented as a pentagon.
Complete Structural Formula Of Cyclopropane
And cyclobutane ( c 4 h 8) by the structural formula. Contact with the liquid may cause frostbite. Web describe, and sketch the conformation of cyclopropane, cyclobutane, and cyclopentane. In addition, cyclopropane has considerable torsional.
Cyclopropane Molecular Model PNG Images & PSDs for Download
The simplest of these cyclic hydrocarbons has the formula c 3 h 6. The parent of the class of cyclopropenes. Note the triangle between the three carbon atoms for cyclopropane, and the square between the.
draw the structure of cyclopropane mandjpainting
Note the triangle between the three carbon atoms for cyclopropane, and the square between the four carbon atoms for cyclobutane. Web name cycloalkanes using iupac (systematic) and selected common name nomenclature. And cyclobutane ( c.
Illustrated Glossary of Organic Chemistry Cyclopropane
Cyclohexane, one of the most common cycloalkanes is shown below as an example. Analyze the stability of cyclobutane, cyclopentane and their substituted derivatives in terms of angular strain, torsional strain and steric interactions. Propose a.
3d model of cyclopropane YouTube
Web when a chain contains three or more carbon atoms, the atoms can join to form ring or cyclic structures. Web draw the structure for each compound. Images of the chemical structure of cyclopropane are.
Complete Structural Formula Of Cyclopropane
(do not draw the hydrogen atoms.) this problem has been solved! It can be represented as a pentagon. The bond angles in cyclopropane are approximately 60 degrees, which is much smaller than the typical bond.
Special Collections & Archives Research Center Pastel drawing of the
Figure 4.5 the structure of cyclopropane, showing the eclipsing of neighboring c. Cyclohexane, one of the most common cycloalkanes is shown below as an example. It is a cycloalkene and a member of cyclopropenes. Web.
Cyclopropane Alchetron, The Free Social Encyclopedia
Web a chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. Web draw the structure for each compound. Describe the bonding in cyclopropane, and use.
draw the structure of cyclopropane 3dartdrawingpencilsketchesawesome
Web draw the structure of cyclopentane. Web draw the structure of cyclopropane this problem has been solved! The molecule has d molecular symmetry. The simplest of these cyclic hydrocarbons has the formula c 3 h.
Medicinal chemistry of Cyclopropane
Web when a chain contains three or more carbon atoms, the atoms can join to form ring or cyclic structures. Web name cycloalkanes using iupac (systematic) and selected common name nomenclature. It can be represented.
Draw The Structure Of Cyclopropane In addition, cyclopropane has considerable torsional strain because the c−h bonds on neighboring carbon atoms are eclipsed ( figure 4.5 ). The chemistry of alkyl halides Web draw the structure for each compound. Web cyclopropane is necessarily planar (flat), with the carbon atoms at the corners of an equilateral triangle. It can asphyxiate by the displacement of air and has a narcotic effect in high concentration (formerly used as an anesthetic gas).