How To Draw A Hydrogen Atom
How To Draw A Hydrogen Atom - Web 5.4k views 7 years ago. Hydrogen, you just need to get to two in that outer shell. The oxygen atom have 2 lone pairs. Web i | ionization constants of weak bases j | solubility products k | formation constants for complex ions l | m | index by the end of this section, you will be able to: Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system.
Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. [2] deuterium ( 2h) contains one neutron and one proton in its nucleus. Web draw tower grating (dtg) with large capacity, long distance, fast response and other advantages is rapidly becoming the current mainstream fiber optic sensors, but want to write high reflectivity fiber on the draw tower fiber is particularly difficult. E ( n) = − 1 n 2 ⋅ 13.6 ev You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these bonds. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Keep in mind to start with the electronegative atoms and proceed to the electropositive one.
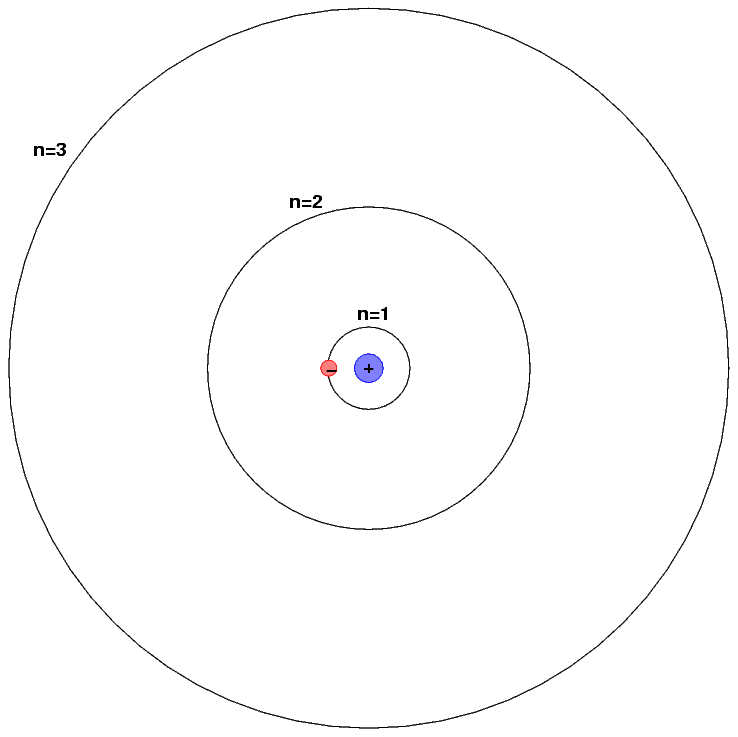
Describe Bohr’s model of the hydrogen atom. bitWise Academy
Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Generally the atom with the highest bonding sites. Web since such a sphere or.
Hydrogen Atom Diagram
But fluorine, you want to get it to eight. Web in general we try to get the octet rule for any atom except for hydrogen. Calculate the total number of valence electrons. Web and to.
Hydrogen Definition, Structure, Properties & Uses Embibe
Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Generally the atom with the highest bonding sites. [2] deuterium ( 2h) contains one neutron and.
How to Learn About the Chemistry of the Hydrogen Atom 12 Steps
[2] deuterium ( 2h) contains one neutron and one proton in its nucleus. Bohr's model calculated the following energies for an electron in the shell, n : Keep in mind to start with the electronegative.
Diagram representation element hydrogen Royalty Free Vector
Here, the given molecule is h2 (or diatomic hydrogen). Take a pen and paper with you and try to draw this lewis structure along with me. Hydrogen is the main component of stars, and a.
Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy
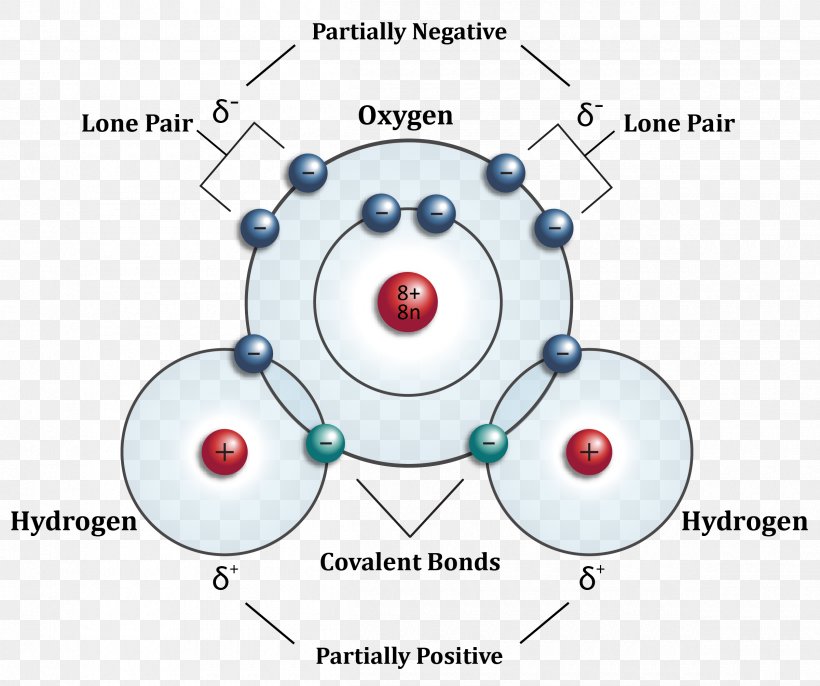
So, if i wrote just the element symbol and its atomic mass on the board that students should be able to figure out the number of particles. Web the oxygen atom (o) is at the.
Hydrogen Atom Diagram
Web the number of dots equals the number of valence electrons in the atom. E ( n) = − 1 n 2 ⋅ 13.6 ev Web the oxygen atom (o) is at the center and.
Diagram Representation Of The Element Hydrogen Stock Vector Image
Calculate the total number of valence electrons. Web i | ionization constants of weak bases j | solubility products k | formation constants for complex ions l | m | index by the end of.
GJ Blogs the atomic structure of hydrogen
Keep in mind to start with the electronegative atoms and proceed to the electropositive one. Each straight line segment represents a bond, the ends and intersections of the lines are carbon atoms, and the correct.
Hydrogen Molecule Diagram
(the order in which the positions are used does not matter.) for example, the lewis electron dot diagram for hydrogen is simply. Generally the atom with the highest bonding sites. The most common element in.
How To Draw A Hydrogen Atom Add enough electrons (dots) to the outer atoms to. So, if i wrote just the element symbol and its atomic mass on the board that students should be able to figure out the number of particles. Web key points bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Web since such a sphere or circle encloses most of (but not all) the electron density, it is about as close as one can come to drawing a boundary which encloses the atom. E ( n) = − 1 n 2 ⋅ 13.6 ev