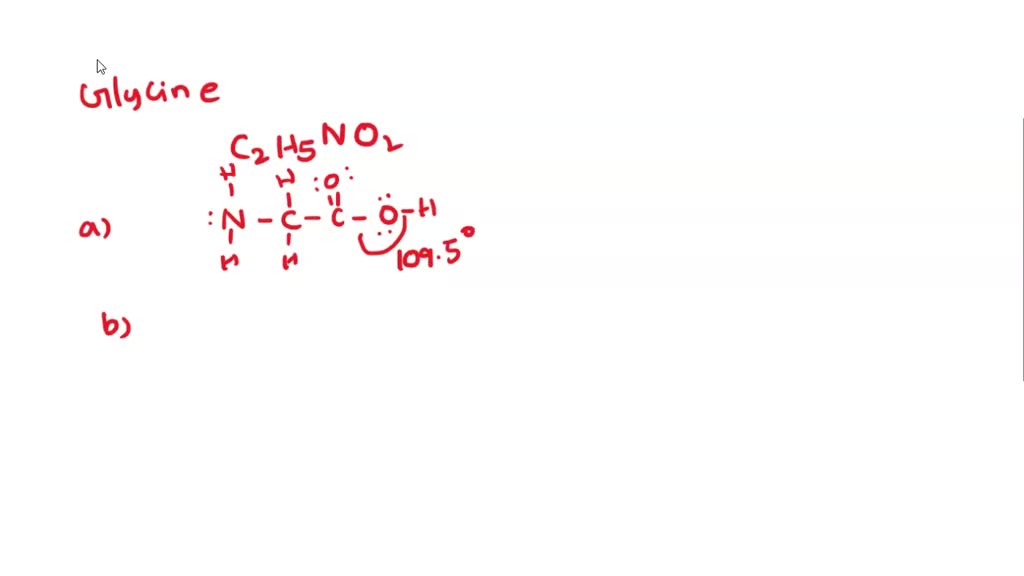Draw An Electron-Dot Structure For Glycine
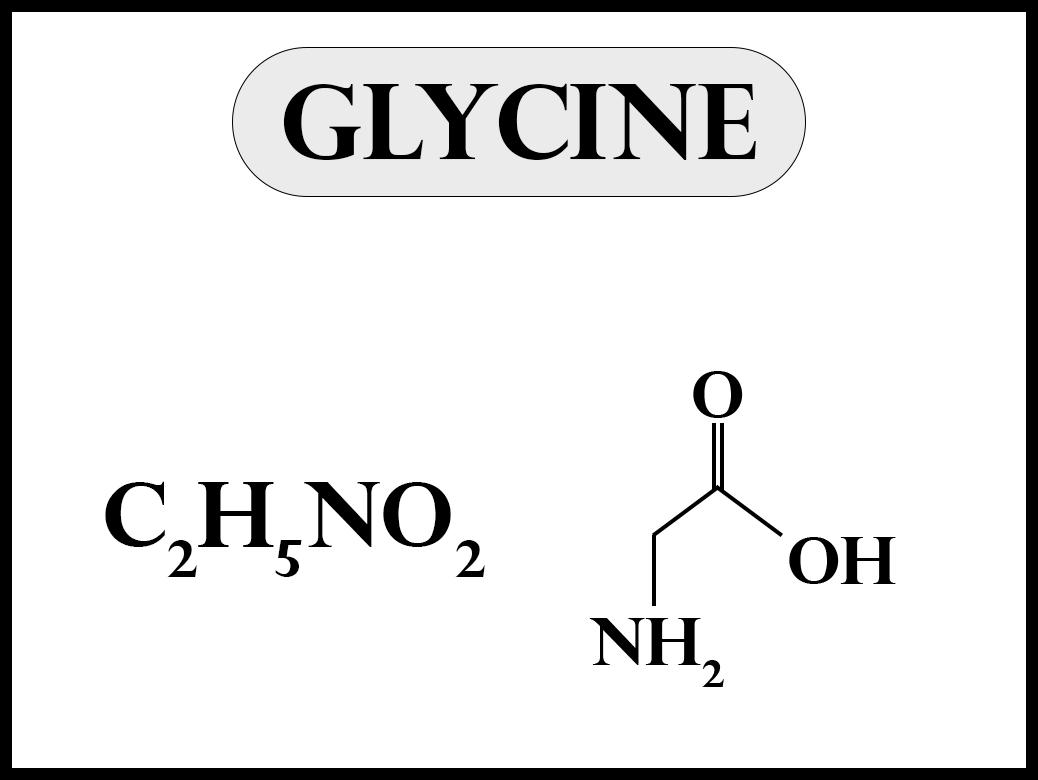
Draw An Electron-Dot Structure For Glycine - Web structural formula and chemical properties. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Also, there is an α carbon at the center that is connected to the amine group, the carboxyl group, and the side chain. Web resonance resonance and dot structures formal charge formal charge and dot structures worked example: Web glycine is an amino acid.
The structures which depict the lone pairs and bonding of atoms in a molecule are called. The lewis structure of glycine is pictured below. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web resonance resonance and dot structures formal charge formal charge and dot structures worked example: It is key in a variety of biological processes. For each of the following, specify the type (s) of bond formed (sigma, o or pi, tl), then specify the orbitals from each atom that overlap to form that bond. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond.
An amino acid that is not optically active is(a) Glycine(b) Valine(c
The simplest amino acid is glycine ( h_2nch_2cooh h 2nc h 2cooh ). Also, there is an α carbon at the center that is connected to the amine group, the carboxyl group, and the side.
Organic Chemistry How To Draw Lewis Structures YouTube
Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web let's recall the structure of glycine. Web chemistry draw lewis dot structure of glycine. Draw the molecule by placing.
15 Lewis Dot Structure Examples Robhosking Diagram
Include all hydrogen atoms and nonbonding electrons. Using formal charges to evaluate nonequivalent resonance structures resonance and formal charge vsepr for 2 electron clouds vsepr for 3 electron clouds more on the dot structure for.
SOLVED The atoms in the amino acid glycine are connected as shown (a
Draw a lewis structure for glycine. Include all lone pairs of electrons and all hydrogen atoms. The structure can be drawn as follows: The number of dots equals the number of valence electrons in the.
How To Draw Lewis Dot Structures » Doubleprogram
In this task, we have to draw the structure of amino acid, glycine. Lone pairs of electrons have been left out; Molecular weight 75.07 g/mol computed by pubchem 2.2 (pubchem release 2021.10.14) dates create: This.
Comment dessiner une représentation de Lewis Wiki Chimie
It may be useful to draw them. Draw the molecule by placing atoms on the grid and connecting them with bonds. This widget gets the lewis structure of chemical compounds. Get the free lewis structure.
3 Ways to Draw Lewis Dot Structures wikiHow
In this task, we have to draw the structure of amino acid, glycine. Web the atoms in the amino acid glycine are connected as shown: Web draw a lewis structure for glycine. Web to draw.
Draw the Lewis dot structure for glycine. Quizlet
Include all lone pairs of electrons and all hydrogen atoms. Web the atoms in the amino acid glycine are connected as shown: Get the free lewis structure finder widget for your website, blog, wordpress, blogger,.
Draw the Lewis dot structure for glycine. Quizlet
The central atoms in the skeletal structure are nitrogen and the two carbon atoms. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web resonance resonance and dot structures formal.
steps for drawing a lewis structure swansonmcarthurphysicaltherapy
Web the atoms in the amino acid glycine are connected as shown: It may be useful to draw them. The central atoms in the skeletal structure are nitrogen and the two carbon atoms. When constructing.
Draw An Electron-Dot Structure For Glycine Web structural formula and chemical properties. Also, there is an α carbon at the center that is connected to the amine group, the carboxyl group, and the side chain. It is key in a variety of biological processes. Include all hydrogen atoms and nonbonding electrons. The structures which depict the lone pairs and bonding of atoms in a molecule are called.