Draw The Electron Configuration For A Neutral Atom Of Iron.
Draw The Electron Configuration For A Neutral Atom Of Iron. - 1s 2 2s 2 2p 6: Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Web in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Locate the nearest noble gas preceding phosphorus in the periodic table. 1s 2 2s 2 2p 3:
Web an electrically neutral atom has the following electron configuration: 1s 2 2s 2 2p 5: Locate the noble gas element in the period above the element of interest. Web so, [ar] can be written instead of 1s^2 2s^2 2p^6 3s^2 3p^6. Web chemistry chemistry questions and answers draw the electron configuration for a neutral atom of iron. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not.
Get the Detailed Periodic table (With Electron Configuration)
Web the electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. We describe an electron configuration with a symbol that contains three pieces of.
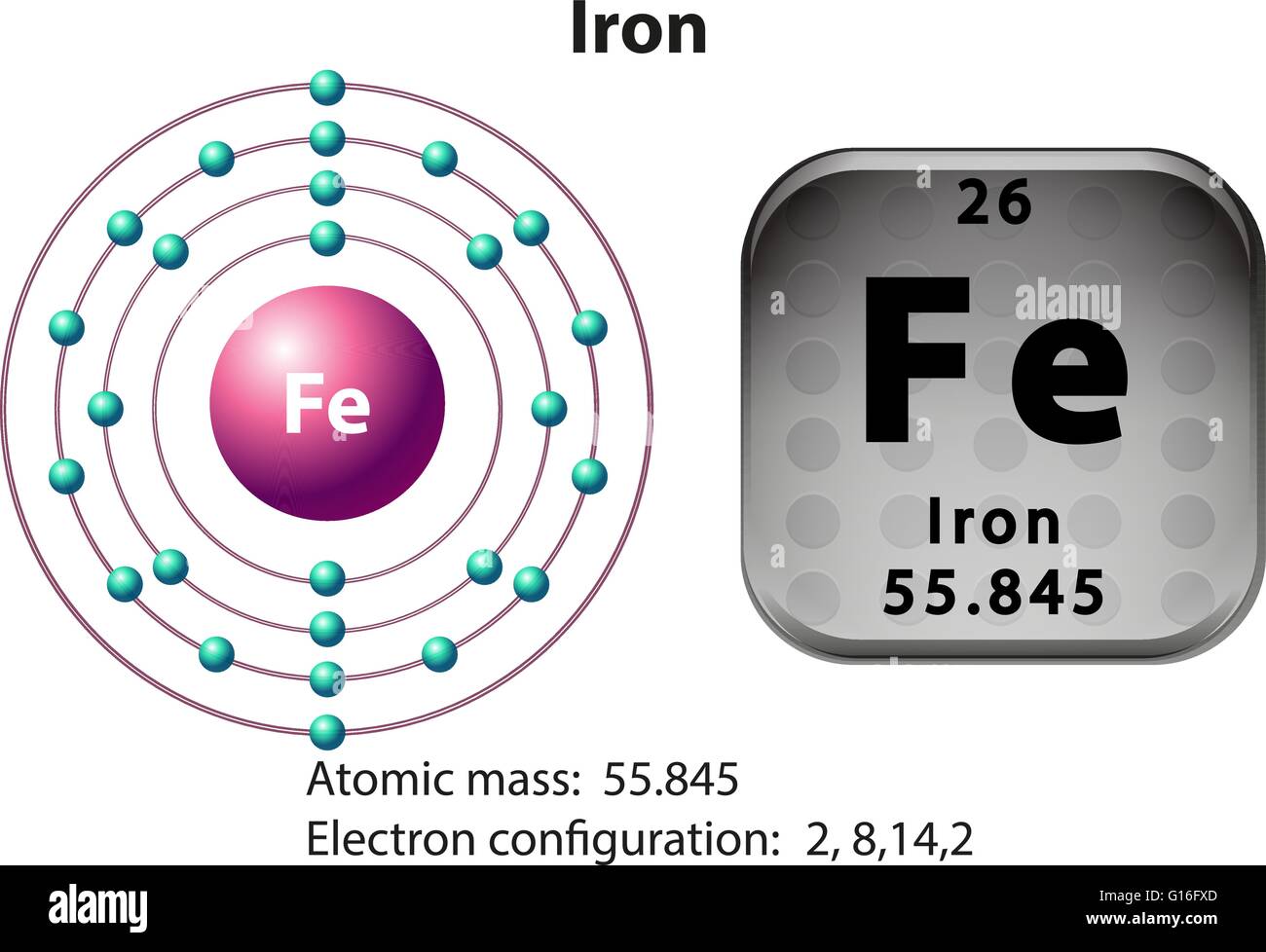
Iron electronic configuration How to Write Iron electronic
The charge is equal to the number of electrons that must be gained to fill the s and p. Remember, a neutral atom contains the same number of protons and electrons. Draw the electron configuration.
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB)
And so first, let's just think about the electron configuration of the simplest element. If we're talking about a neutral hydrogen atom, a neutral hydrogen atom, it has an atomic number of one which tells.
Symbol and electron diagram for Iron illustration Stock Vector Image
Thus, it is simple to determine the charge on such a negative ion: This is sometimes called the bohr, or the ‘solar system’, model. Electrons are represented by dots or crosses and are positioned in.
Solved Draw the electron configuration for a neutral atom of
Every line in the energy diagram below holds 2 electrons of opposite. Because there are no unpaired electrons, zn atoms are diamagnetic. Orbital diagram and valence electron configuration for phosphorus. To facilitate our understanding of.
Flashcard iron with atomic mass Royalty Free Vector Image
1s 2 2s 2 2p 4: Electron configuration of neon (ne) [he] 2s 2 2p 6: The electron configuration of iron is [ ar] 3d 6 4s 2 , if the electron arrangement is through.
Draw the electron configuration for a neutral atom of iron. Quizlet
Step 1/2 the atomic number of iron is. Continue the electron configuration from the noble gas until you reach the element of interest. Web the arrangement of electrons in the orbitals of an atom is.
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration
Web the electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Remember, a neutral atom contains the same number of protons and electrons. We.
Atoms Diagrams Electron Configurations of Elements
Electron configuration of fluorine (f) [he] 2s 2 2p 5: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Determine whether the substance is paramagnetic or diamagnetic. Electrons.
Iron Protons Neutrons Electrons Electron Configuration
#1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. Put the noble gas in brackets and write the remainder of the electron configuration. Web the upper right side shows the number of electrons in a neutral atom. Note.
Draw The Electron Configuration For A Neutral Atom Of Iron. Web in order to write the electron configuration for iron (fe) we first need to know the number of electrons for the fe atom (there are 26 electrons). Web and to help us with that, we will look at a periodic table of elements. Determine whether the substance is paramagnetic or diamagnetic. 1s 2 2s 2 2p 3: 1s 2 2s 2 2p 6:







:max_bytes(150000):strip_icc()/Iron-58b602243df78cdcd83d3d5a.jpg)
