Draw The Lewis Structure For Nf3.
Draw The Lewis Structure For Nf3. - Dive into detailed drawings that reveal the lewis structure of nf3, understand its hybridization, and explore its molecular shape. Draw the structure for bf3. V = 7, b = 2, n = 6. Web 6 steps to draw the lewis structure of nf3 step #1: Using formal charges to determine how many bonds to make, a different perspective.
Draw the structure for bf3. The lewis structure for nf3 has a total of 10 lone pairs. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. All of the above none of the above in writing the lewis structure. This problem has been solved! Web draw the lewis structures for nf3 and if3. Web lewis structure of nf 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps.
Solved Draw the Lewis structure of NF3. +
We also discuss the distribution of charges and electron pairs in this fascinating molecule. Dive into detailed drawings that reveal the lewis structure of nf3, understand its hybridization, and explore its molecular shape. Tetrahedral bf,.
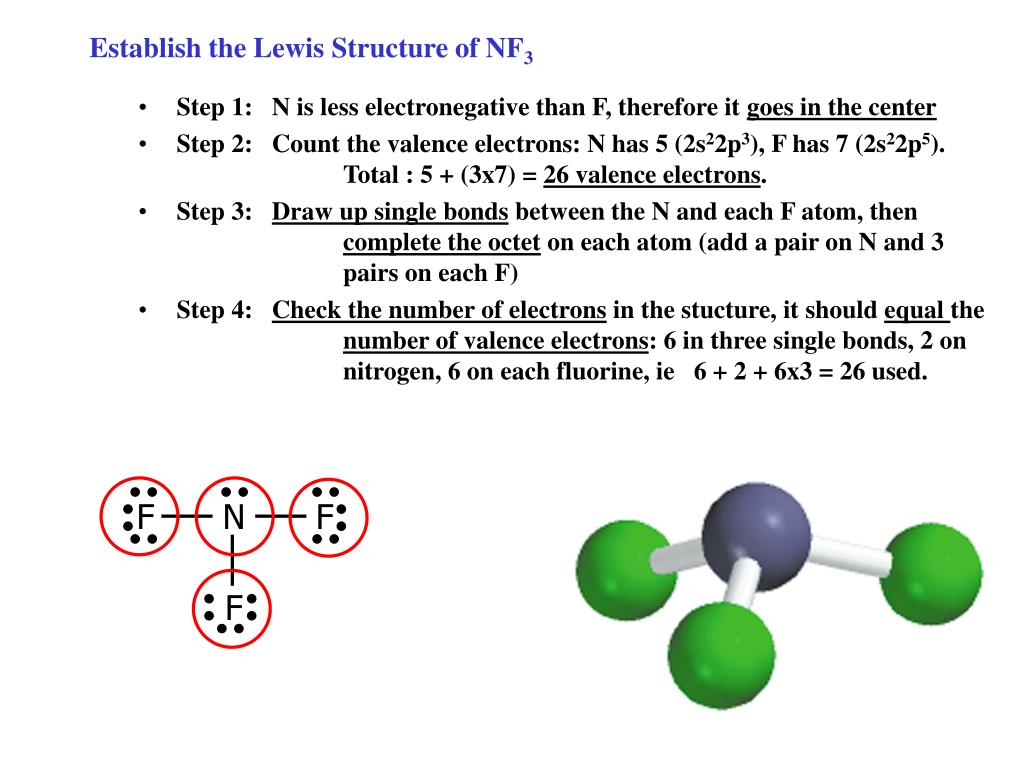
PPT Establish the Lewis Structure of NF 3 PowerPoint Presentation
Web drawing lewis structures for molecules with one central atom: The octet rule is satisfied for all atoms. Web by using the following steps, you can easily draw the lewis structure of nf 3: Web.
draw lewis structures for the following molecules nf3 csnplacement
The lewis structure for nf3 has 3 bonding pairs. Web by pomila sharma unlock the complexities of nf3 (nitrogen trifluoride) with our comprehensive blog post. After drawing the lewis structure of nf 3, you can.
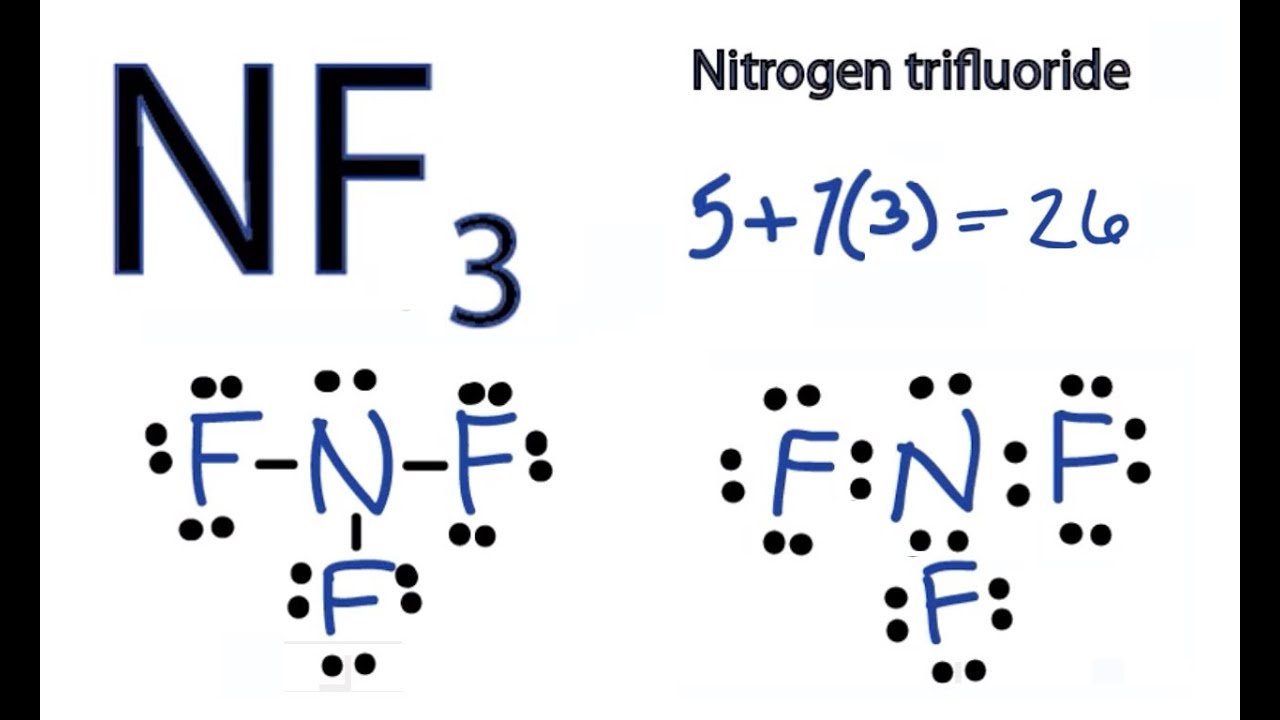
NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
Web 6 steps to draw the lewis structure of nf3 step #1: Tetrahedral bf, and nf, both have three covalently bonded fluorine atoms around a central atom. You'll get a detailed solution from a subject.
NF3 Lewis Structure How to Draw the Dot Structure for NF3 (Nitrogen
N (group 15) has 5 valence electrons, and f (group 17) has 7 valence electrons. All of the above none of the above in writing the lewis structure. Here, the given molecule is nf3 (nitrogen.
lewis structure for nitrogen trifluoride
Web drawing lewis structures for molecules with one central atom: Draw the lewis structure for nf3 (nitrogen is the central atom) and select the correct model representing its electron pair geometry and molecular geometry electron.
So far, we’ve used 26 of the NF3 Lewis structure’s total 26 outermost
Dive into detailed drawings that reveal the lewis structure of nf3, understand its hybridization, and explore its molecular shape. We also discuss the distribution of charges and electron pairs in this fascinating molecule. In the.
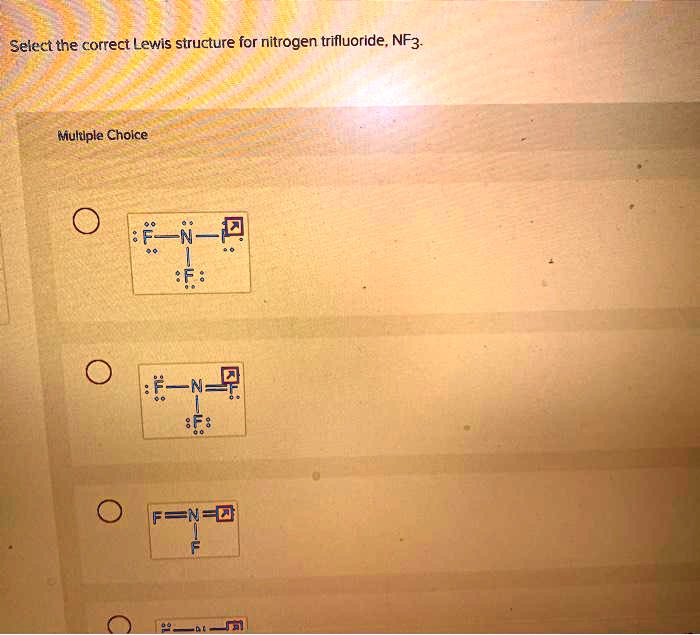
SOLVED Select the correct Lewis structure for nitrogen trifluoride
Web the first step is to sketch the lewis structure of the nf3 molecule, to add valence electrons around the nitrogen atom; Part 2 (1 point) what is the molecular geometry for nf3? Get the.
NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
Dive into detailed drawings that reveal the lewis structure of nf3, understand its hybridization, and explore its molecular shape. Using formal charges to determine how many bonds to make, a different perspective. Draw the lewis.
Draw the Lewis structure for NF3 What are its electron pair and
The second step is to add valence electrons to the three fluorine atoms, and the final step is to combine the. Web by using the following steps, you can easily draw the lewis structure of.
Draw The Lewis Structure For Nf3. Web nf3 lewis structure. V = 7, b = 2, n = 6. The lewis structure for nf3 has a total of 10 lone pairs. By following these steps, you have successfully drawn the lewis structure of nf3, ensuring that it adheres to the principles of valence. Web view the full answer.