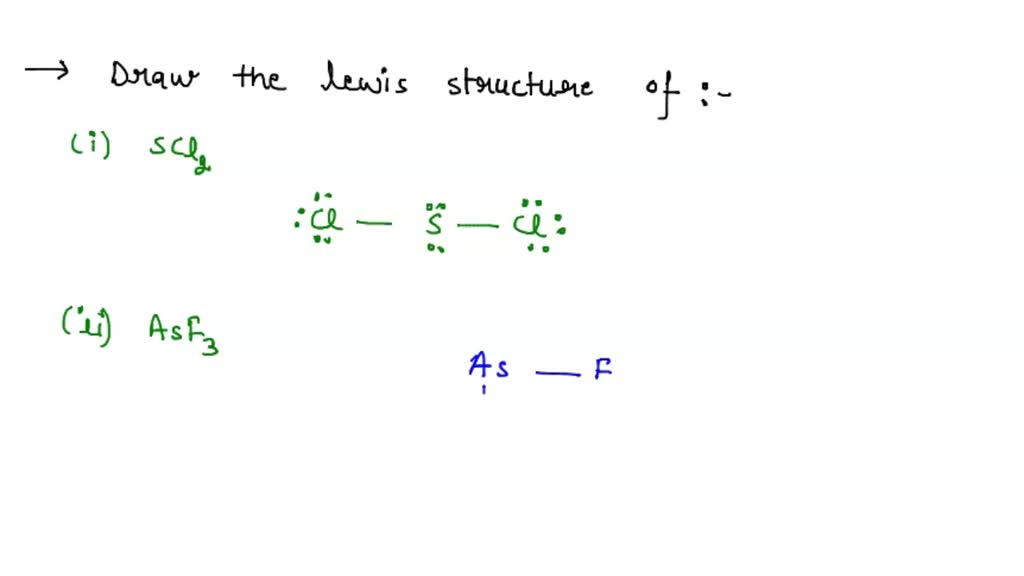Draw The Lewis Structure For Scl2
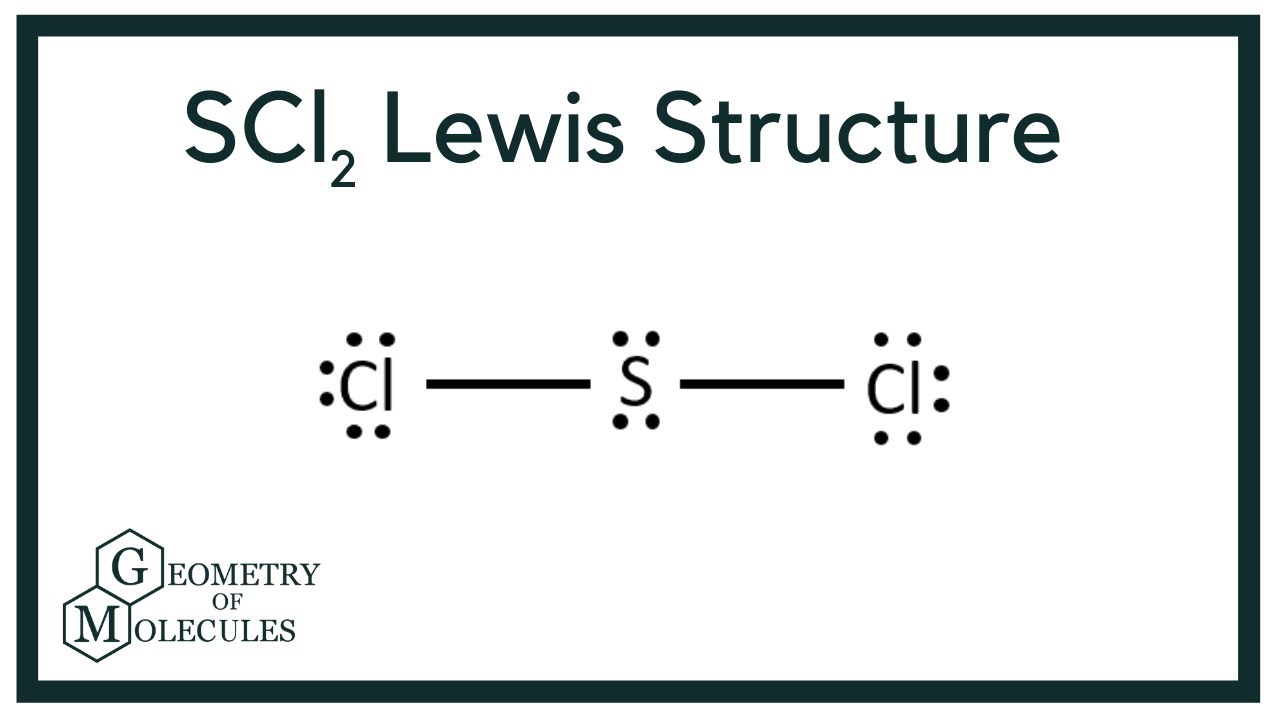
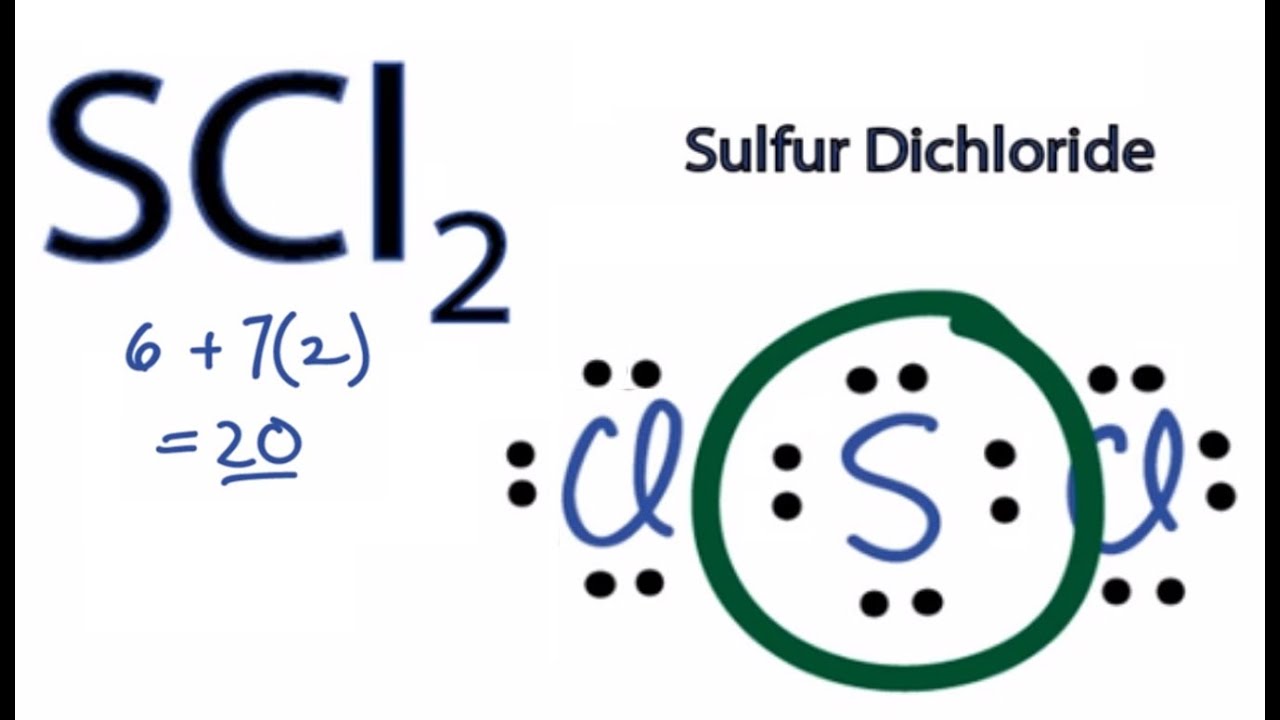
Draw The Lewis Structure For Scl2 - Web to draw the lewis structure for scl2 (sulfur dichloride), we need to determine the valence electrons in scl2. Web draw the lewis structure for scl 2 and use it to identify the correct statements that follow. [ ] the central atom electron geometry is tetrahedral. Six plus 14 equals 20 valence electrons. Chlorine is in group 7, so it has 7 valence electrons.
But we have two chlorines so let's multiply that by 2. Because scl 2 is an simple molecule and contains only three atoms, drawing its lewis structure is not a challenging one. Web watch on see the big list of lewis structures transcript: Web 6 steps to draw the lewis structure of scl2 step #1: Drawing the lewis structure for scl 2 video: Draw the lewis electron dot structure for scl2 and discuss its molecular geometry. Web to properly draw the scl 2 lewis structure, follow these steps:
Is SCl2 Polar or Nonpolar? Techiescientist
Chlorine is in group 7, so it has 7 valence electrons. Here, the given molecule is scl2 (sulfur dichloride). On the periodic table, sulfur—group 6 or 16—has 6 valence electrons. Drawing the lewis structure for.
SOLVED Draw the Lewis structure for SCl2. How many lone pairs of
Web drawing lewis structures for molecules with one central atom: Calculate the total number of valence electrons. Chlorine, group 7 or 17 has 7; Web to draw the lewis structure for scl2 (sulfur dichloride), we.
Lewis Dot Structure For Scl2 Draw Easy
The second step is to add valence electrons to the two chlorine atoms, and the final step is to combine the. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal.
SOLVED 1) Draw the Lewis structure of A) SCl2 B) AsFr3 2) Using only
Web chemistry chemistry questions and answers draw the lewis structure for scl2 in the marvin window below and then answer the questions that follow. The following procedure can be used to construct lewis electron structures.
SCl2 Molecular Geometry Science Education and Tutorials
Use your molecular models to explain why this molecule is polar. The second step is to add valence electrons to the two chlorine atoms, and the final step is to combine the. The following procedure.
SCl2 Lewis Structure, Geometry, Hybridization, and Polarity
For the scl2 structure use the periodic table to find the total number of valence electrons. Scl2 is used to synthesize. Web scl2 is unstable and exists in equilibrium with s2cl2, as shown. Figure out.
So far, we’ve used 20 of the SCl2 Lewis structure’s total 20 outermost
On the periodic table, sulfur—group 6 or 16—has 6 valence electrons. [ ] the molecule is nonpolar. Web the first step is to sketch the lewis structure of the scl2 molecule, to add valence electrons.
SCl2 (Sulfur dichloride) Molecular Geometry, Bond Angles & Electron
It won't allow me to upload images. While selecting the atom, always put the least electronegative atom at the center. (b) what is the the shape (molecular geometry) of scl2? I also go over hybridization,.
Lewis Structure of SCl2 (sulfur dichloride) YouTube
Chlorine is in group 7, so it has 7 valence electrons. You'll get a detailed solution from a subject matter expert that. Scl2 is used to synthesize. Number of steps can be changed according the.
SCl2 Lewis Structure How to Draw the Lewis Structure for SCl2 YouTube
[ ] the molecule is nonpolar. Calculate the total number of valence electrons. Web to draw the lewis structure for scl2 (sulfur dichloride), we need to determine the valence electrons in scl2. Number of steps.
Draw The Lewis Structure For Scl2 Web this problem has been solved! #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail. I can't draw it but each atom has 6 electrons around it. #1 draw a rough sketch of the structure first, determine the total number of valence electrons The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom.