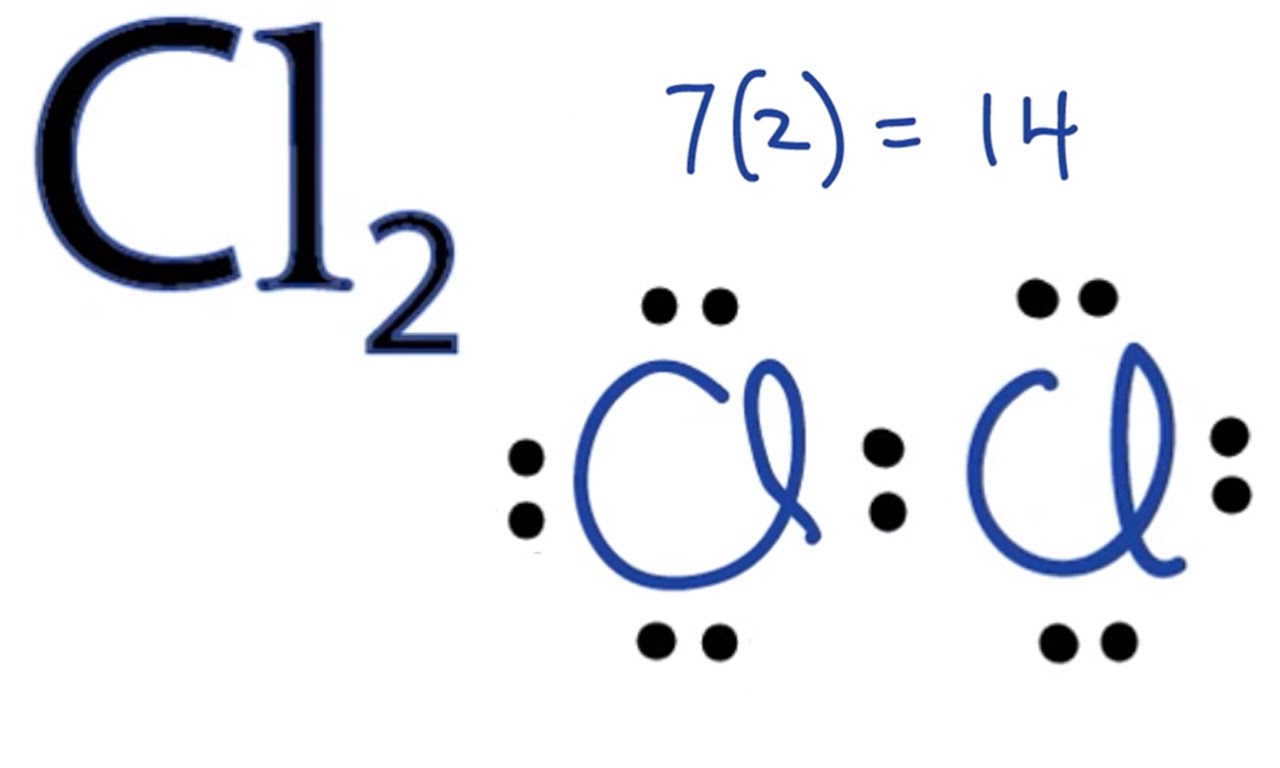Draw The Orbital Diagram For Chlorine
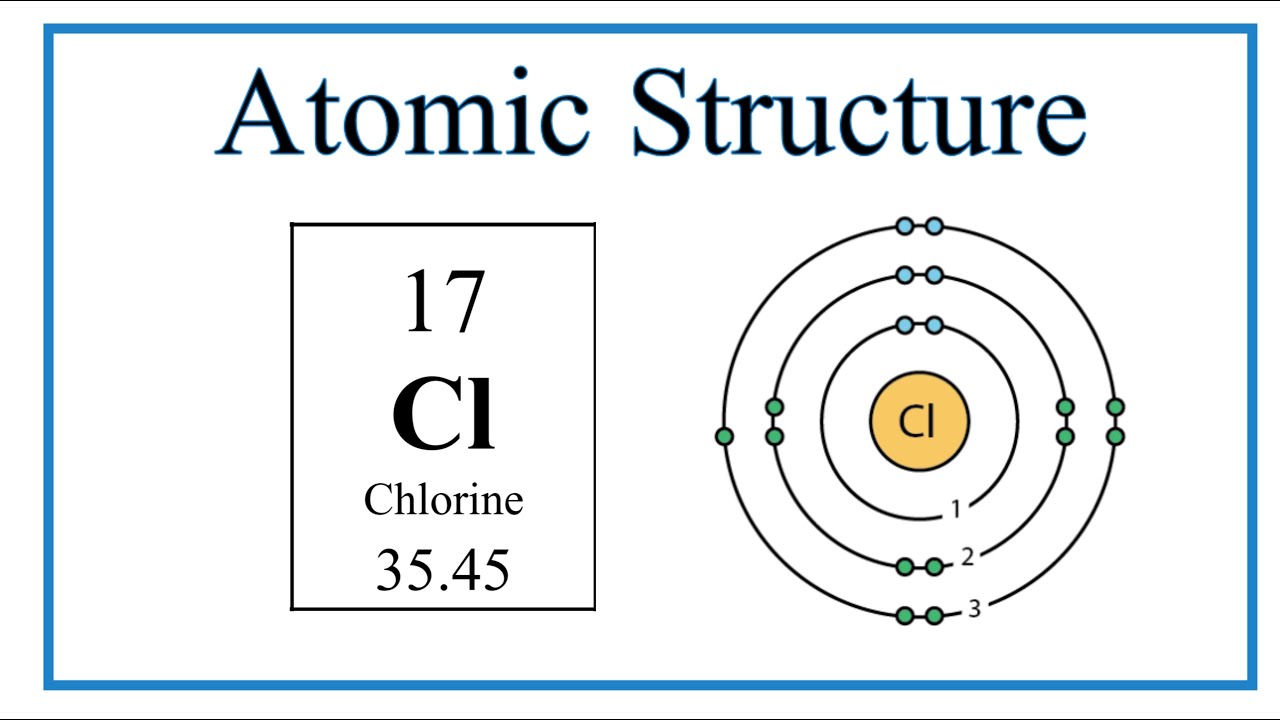
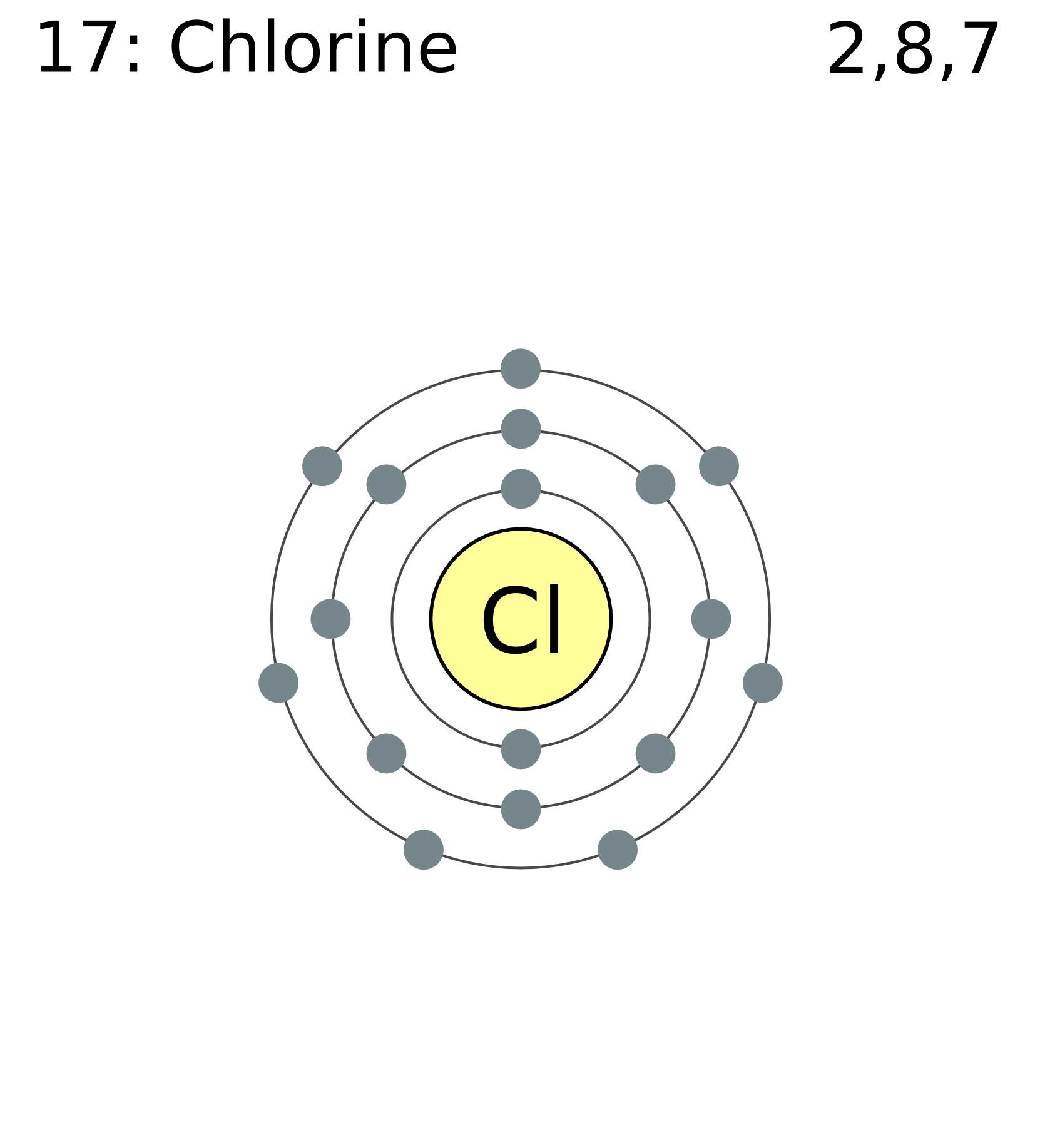
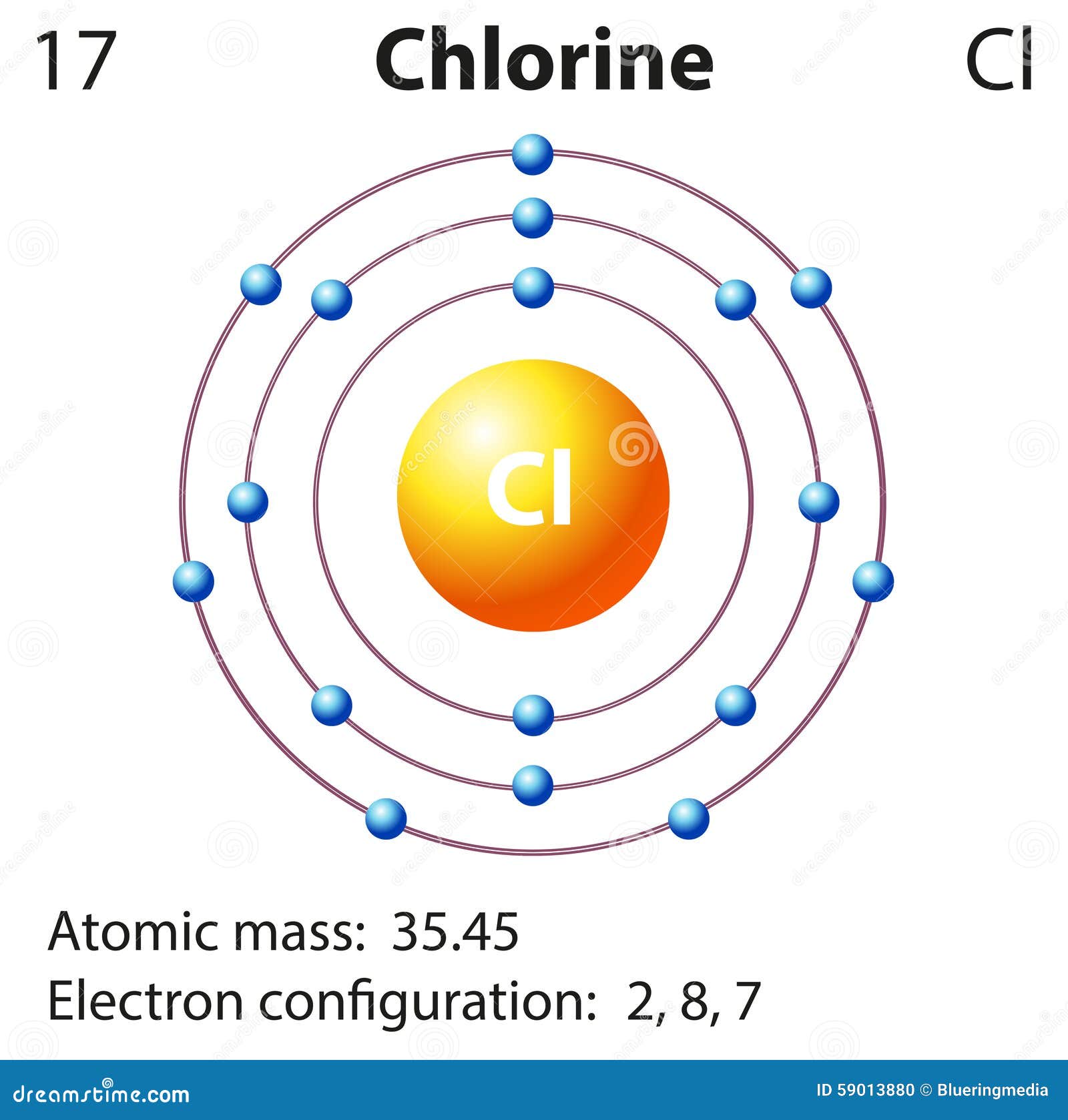
Draw The Orbital Diagram For Chlorine - If there is only one orbital needed, but three boxes provided, make sure to enter your orbital/answer in the middle box. Web to visualize the electron configuration of chlorine, we can use an orbital diagram. Orbital is the region of space around the nucleus of an atom where electrons are found. Web electron configuration diagram for chlorine. Web the outermost shell in the bohr diagram of chlorine contains 7 electrons that also called valence electrons.
Web electron configuration diagram for chlorine. To do that we need to find the number. Bohr model describes the visual representation of orbiting electrons around the small nucleus. Since 1s can only hold two electrons the next 2 electrons for chlorine go in the 2s orbital. Web in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. Now in the next step,. Each sublevel is labeled by its shell and sublevel.
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube
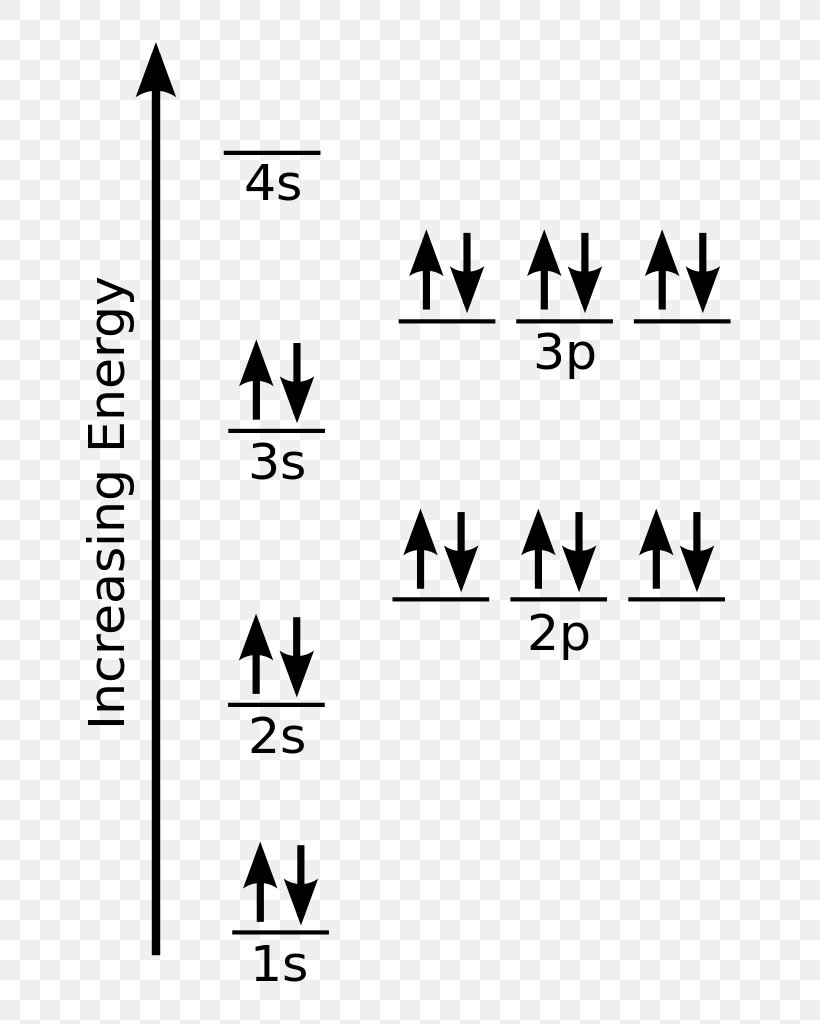
Web an orbital box diagram can be written as well. The orbital box diagrams are listed for the first 20 elements in the figure below. The orbitals are 1s, 2s, 2p, 3s, and 3p. For.
Electron Configuration Atomic Orbital Chlorine Chemistry, PNG
This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. Web fluorine (atomic number 9) has only one 2p orbital containing an unpaired.
Chlorine electron configuration Stock Image C029/5025 Science
The atomic number of chlorine represents the total number of electrons of chlorine. That's 2 in the first shell (full) and then 8 in the second shell (also full) show more. In the third column,.
Chlorine Electron Configuration (Cl) with Orbital Diagram
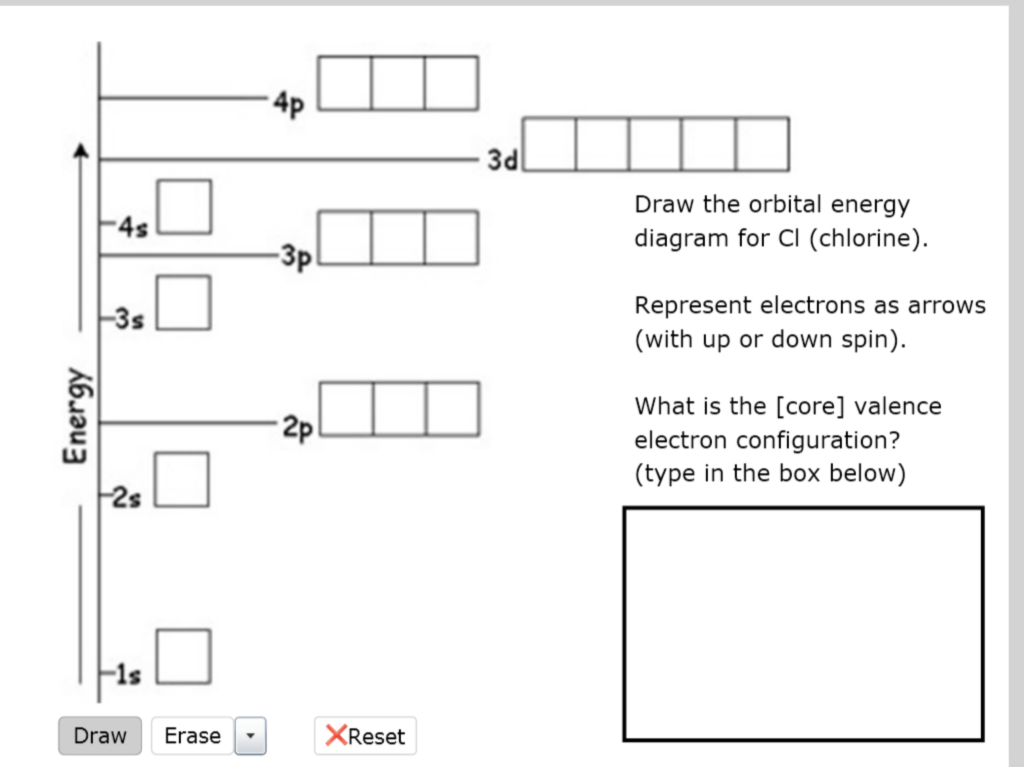
This diagram shows how the electrons in the chlorine atom are arranged in different orbitals. Determine the following (write the number answer only) number of unpaired electrons = number of valence electrons = number of.
FileElectron shell 017 chlorine.png Wikimedia Commons
Web to write the orbital diagram for the chlorine atom (cl) first we need to write the electron configuration for just cl. The next six electrons will go in the 2p orbital. An orbital diagram.
Solved 4p 3d Draw the orbital energy diagram for Cl
Web what is the orbital diagram for chlorine (cl)? Web chemistry questions and answers. Web to write the orbital diagram for the chlorine atom (cl) first we need to write the electron configuration for just.
Electron Configuration For Chlorine
Each sublevel is labeled by its shell and sublevel. Web steps find electrons. The orbitals are 1s, 2s, 2p, 3s, and 3p. Web science chemistry chemistry questions and answers write the electron configuration for a.
Diagram Representation of the Element Chlorine Stock Illustration
The p, d, and f orbitals have different sublevels, thus can hold more electrons. Web the orbital diagram or orbital notation simply represents the arrangement of electrons in different orbitals of an atom. In an.
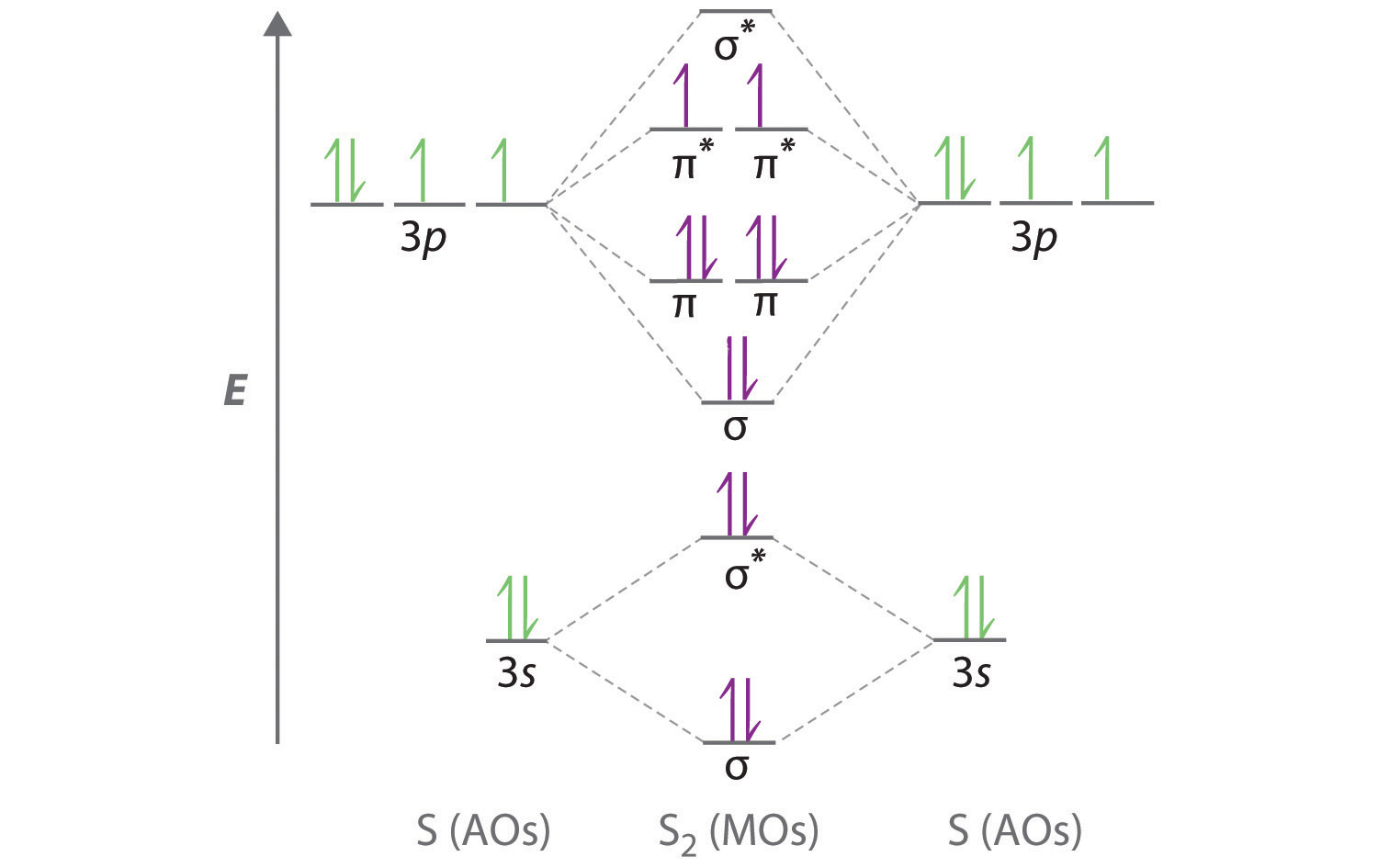
Molecular Orbital Diagram For Cl2
If there is only one orbital needed, but three boxes provided, make sure to enter your orbital/answer in the middle box. Determine the following (write the number answer only) number of unpaired electrons = number.
Chlorine Cl (Element 17) of Periodic Table NewtonDesk
In an orbital diagram, an electron is represented by an arrow, while a box represents an atomic orbital. Bohr model describes the visual representation of orbiting electrons around the small nucleus. Web the orbital diagram.
Draw The Orbital Diagram For Chlorine We start with a single hydrogen atom (atomic number 1), which consists of one proton and one electron. Determine the following (write the number answer only) number of unpaired electrons = number of valence electrons = number of core electrons = this problem has been solved! This diagram shows how the electrons in the chlorine atom are arranged in different orbitals. The orbital diagram for chlorine would show the filling of the 1s, 2s, 2p, 3s, and 3p orbitals according to its electron configuration. Page contents show how to draw bohr model of chlorine (cl)?