Identifying The Limiting Reactant In A Drawing Of A Mixture
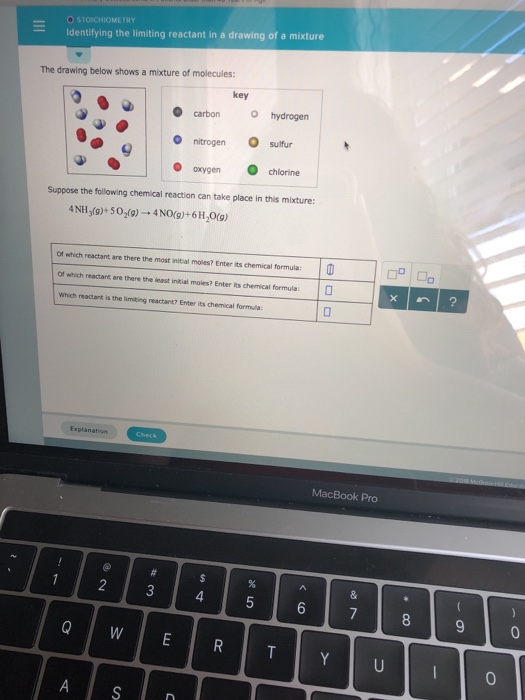
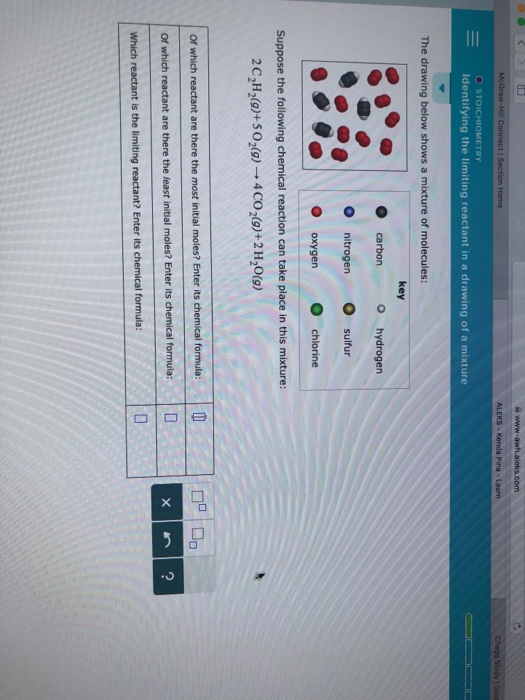
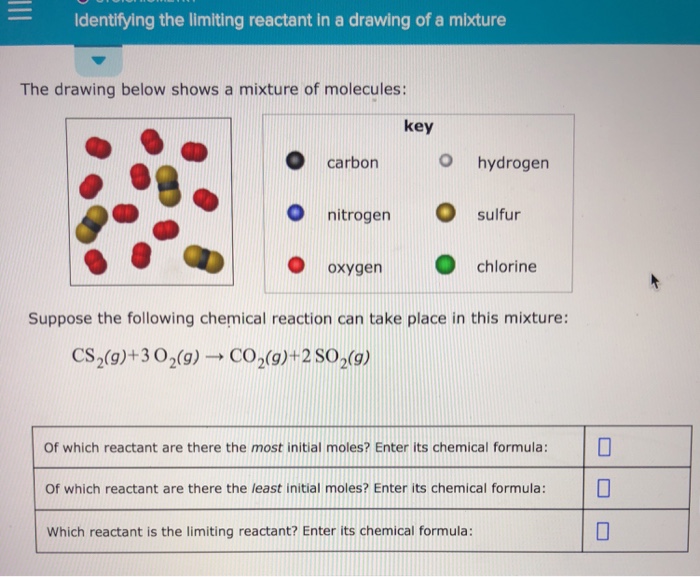
Identifying The Limiting Reactant In A Drawing Of A Mixture - Ch4(g)+2o2(g)→co2(g)+2h2o(g) of which reactant are there. Calculate the mass of excess reactant that reacts. Whichever reactant gives the lesser amount of product is the limiting reagent. Web enter its chemical formula: Key | 0 o carbon hydrogen o nitrogen sulfur o oxygen chlorine suppose the following chemical reaction can take place in this mixture:
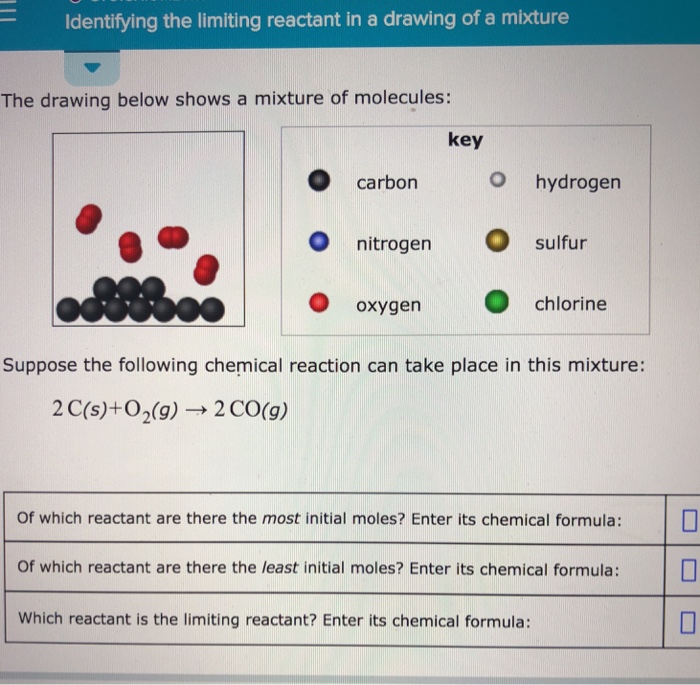
Web identify the limiting reactant(s) and excess reactant(s). Stoichiometry identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: Which reactant is the limiting reactant? Convert all given information into moles. Web try it free. Web m o chemical reactions identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: Carbon nitrogen oxygen key o hydrogen sulfur chlorine suppose the following chemical reaction can take place in this mixture:
(Get Answer) STOICHIOMETRY ? Identifying The Limiting Reactant In A
Web the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Key | 0 o carbon hydrogen o nitrogen sulfur o oxygen chlorine suppose the.
Aleks Identifying the limiting reactant in a drawing of a mixture YouTube
Whichever reactant gives the lesser amount of product is the limiting reagent. Stoichiometry identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: What we need to.
How To Find The Limiting Reactant In A Chemical Reaction?
Ch4(g)+2o2(g)→co2(g)+2h2o(g) of which reactant are there. Key | 0 o carbon hydrogen o nitrogen sulfur o oxygen chlorine suppose the following chemical reaction can take place in this mixture: Count the number of particles in.
ALEKS Identifying the Limiting Reactant in a Drawing of a Mixture
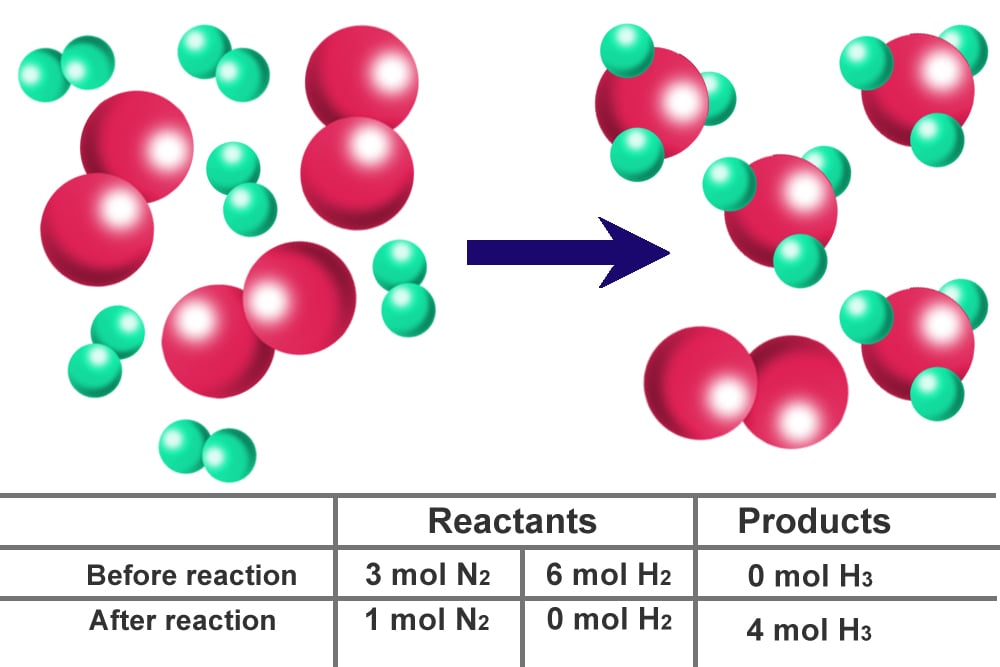
It is first necessary to convert the given quantities of each reactant to moles in order to identify the limiting reactant. Which reactant is the limiting reactant? Calculate the mole ratio from the given information..
Solved O STOICHIOMETRY Identifying The Limiting Reactant
Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Your answer is incorrect the drawing below shows a mixture.
(Solved) Identifying The Limiting Reactant In A Drawing Of A Mixture
Key o hydrogen carbon sulfur o nitrogen chlorine oxygen suppose the following chemical reaction can take place in this mixture: Your answer is incorrect the drawing below shows a mixture of molecules: Key o hydrogen.
5.3b Identifying the limiting reactant in a drawing of a mixture YouTube
What we need to do is determine an amount of one product (either moles or mass) assuming all of each reactant reacts. Suppose the following chemical reaction can take place in this mixture: Web the.
(Solved) Identifying The Limiting Reactant In A Drawing Of A Mixture
Which reactant is the limiting reactant? To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the balanced chemical.
ALEKS Identifying the Limiting Reactant in a Drawing of a Mixture
Suppose the following chemical reaction can take place in this mixture: Web high school chemistry skills practice 1. Web identify the limiting reactant(s) and excess reactant(s). Stoichiometry 一 identifying the limiting reactant in a drawing.
How to Identify the Limiting Reactant in a Drawing of a Mixture
Stoichiometry identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: Stoichiometry identifying the limiting reactant in a drawing of a mixture the drawing below shows a.
Identifying The Limiting Reactant In A Drawing Of A Mixture Web identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: Ch4(g)+2o2(g)→co2(g)+2h2o(g) of which reactant are there. O o carbon nitrogen oxygen key o hydrogen o sulfur chlorine suppose the following chemical reaction can take place in this mixture: What we need to do is determine an amount of one product (either moles or mass) assuming all of each reactant reacts. Stoichiometry identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: