P Orbital Drawing
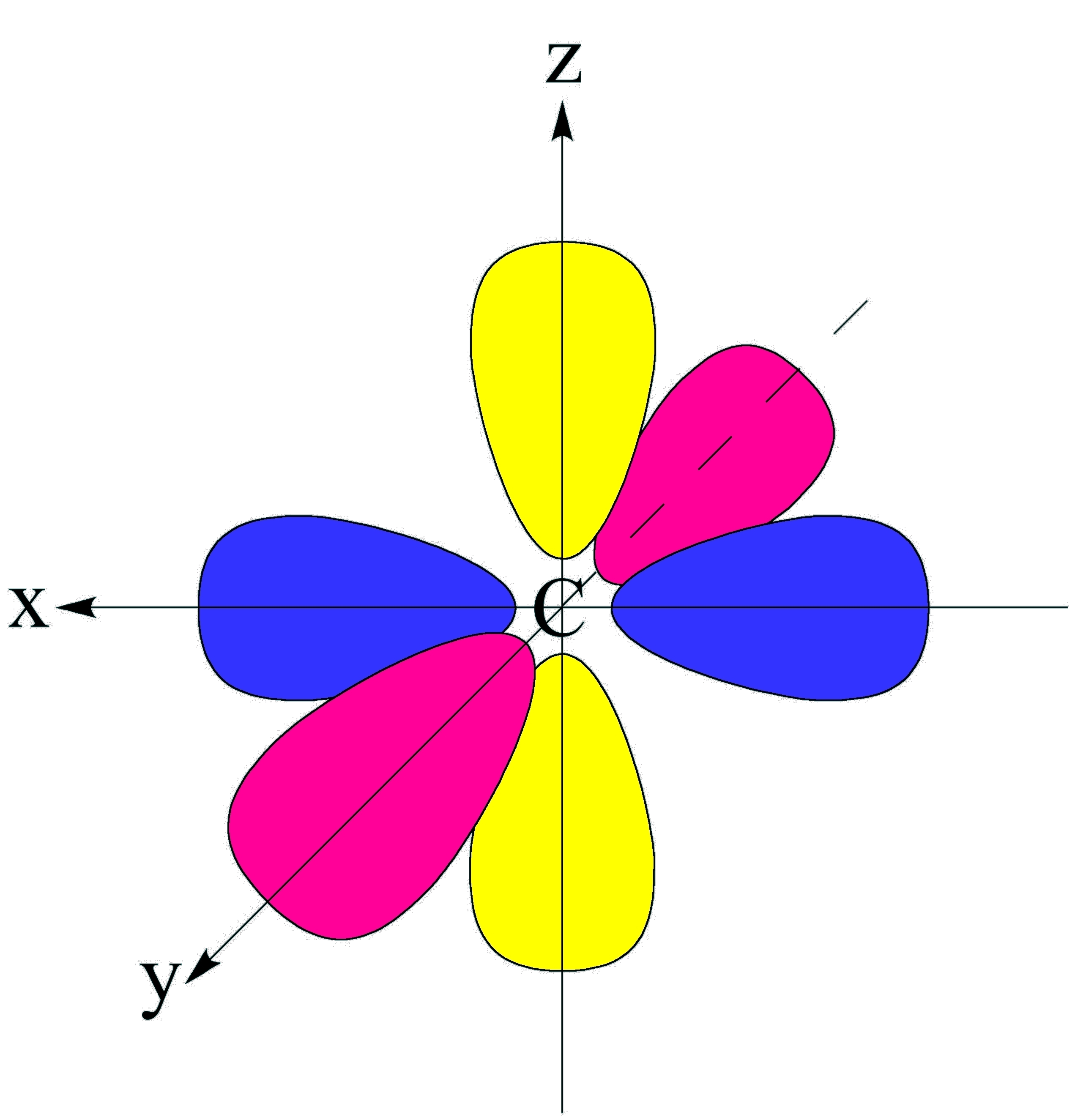
P Orbital Drawing - The p sub shell can hold a maximum of six electrons as there are three orbitals within this sub shell. Because the 2 p subshell has l = 1, with three values of ml (−1, 0, and +1), there are three 2 p orbitals. A smaller sized orbital means there is a greater chance. Web the shapes of p, d and f orbitals are described verbally here and shown graphically in the orbitals table below. P x, p y & p z was this answer helpful?
Web the shapes of p, d and f orbitals are described verbally here and shown graphically in the orbitals table below. Web p orbitals (l=1) only s orbitals are spherically symmetrical. Imagine a horizontal plane through the nucleus, with one lobe of the orbital above the plane and the other beneath it; Web there are three degenerate 2p orbitals (m l = −1, 0, +1) and the electron can occupy any one of these p orbitals. So let's say that that's the nucleus and i'll just draw their p orbitals. A smaller sized orbital means there is a greater chance. Web 21,441 according to the quantum atomic model, an atom can have many possible numbers of orbitals.
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry
This type of hybridization is required whenever an atom is surrounded by three groups of electrons. Web 21,441 according to the quantum atomic model, an atom can have many possible numbers of orbitals. Notice that.
quantum chemistry How do 1s and 2p orbitals overlap? Chemistry
So let's say that that's the nucleus and i'll just draw their p orbitals. So a p orbital is just that dumbbell shape. Label the positions of the oxygen nuclei with the symbol o. In.
How To Draw Orbitals Deepcontrol3
Web what is the orbital diagram for phosphorus (p)? P x, p y & p z was this answer helpful? Web 1) draw each orbital superimposed on a labeled coordinate system (i.e. This type of.
Describe the shapes of s and p orbitals.
A smaller sized orbital means there is a greater chance. This is also due to the history when they were discovered. Propanone (d) draw the electron dot structure for: Web there are three degenerate 2p.
Shapes of Atomic Orbitals — Overview & Examples Expii
The p sub shell can hold a maximum of six electrons as there are three orbitals within this sub shell. Those electrons can participate in resonance. P x, p y & p z was this.
Shape of porbitals in 3D
Web solution verified by toppr p orbital has 3 orientations: Because the 2 p subshell has l = 1, with three values of ml (−1, 0, and +1), there are three 2 p orbitals. Those.
2. What is the shape of p orbital? Brainly.ph
A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals. So let's say that that's the nucleus and i'll just draw their p orbitals. The.
Atomic orbitals explained polizhuge
Web an orbital is a space where a specific pair of electrons can be found. Web if that nitrogen is sp two hybridized, that nitrogen has a p orbital, so we can go ahead and.
Illustrated Glossary of Organic Chemistry Orbital
Web p orbitals (l=1) only s orbitals are spherically symmetrical. Imagine a horizontal plane through the nucleus, with one lobe of the orbital above the plane and the other beneath it; As the value of.
[Solved] sketch sigma and pi bond from p orbital Course Hero
The orbital diagram for phosphorus is drawn with 5 orbitals. Web solution verified by toppr p orbital has 3 orientations: 22 similar questions q 1 (a) draw the electron dot structure for: A fundamental principle.
P Orbital Drawing Those electrons can participate in resonance. Because the 2 p subshell has l = 1, with three values of ml (−1, 0, and +1), there are three 2 p orbitals. Web what is the orbital diagram for phosphorus (p)? P x, p y & p z was this answer helpful? These orbitals can be categorized on the basis of their size, shape or orientation.